In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
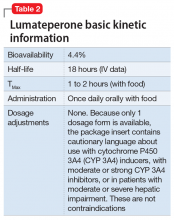
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line