Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
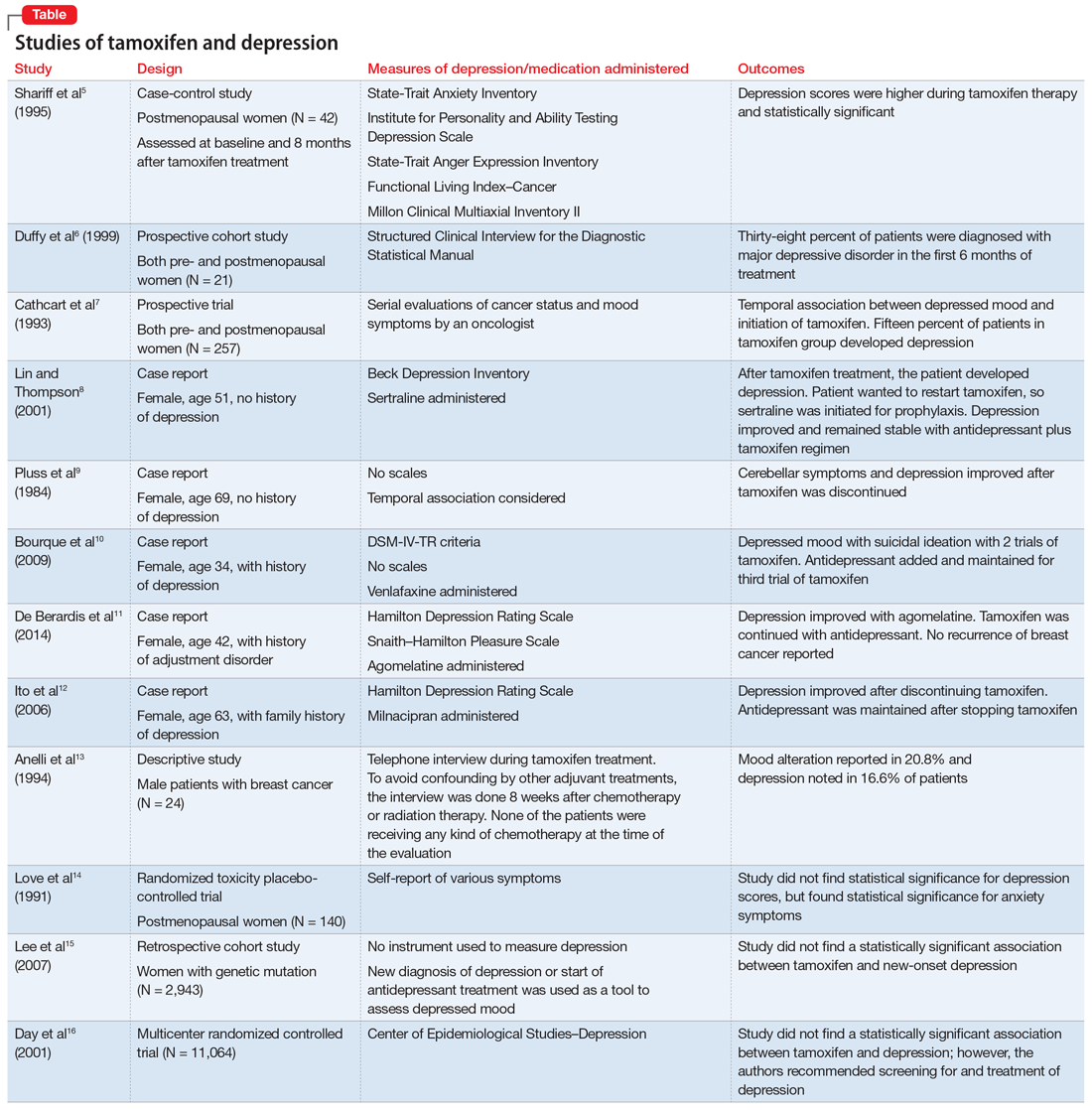
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6
In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...