Marijuana has long been known to stimulate appetite, particularly for sweet foods.1 The naughty boys in my fraternity called it “the munchies;” the professionals call it hyperphagia. Cannabinoid receptor (CB1) stimulation by marijuana’s main active component—9-THC—is believed to induce this behavior. Clinicians have successfully used this effect to treat AIDS-related wasting syndrome and other anorexic conditions.2
CB1 is widely expressed throughout the brain and seems to inhibit release of various neurotransmitters.3 How this effect leads to increased appetite is unclear, but it may result from a decrease in the appetite-suppressing effects of hormones such as leptin. In other words, tweaking the CB1 receptor may take the “brakes” off appetite.
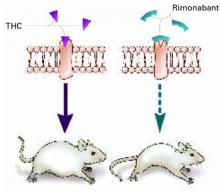
Some researchers have speculated that if stimulating CB1 triggers appetite, blocking the receptor might inhibit it (Figure 1).
THE ‘MUNCHIES’ IN MICE
Rimonabant (SR141716), an experimental agent, is a potent and selective CB1 antagonist.
Ravinet Trillou et al fed mice a high-fat diet known to induce obesity.4 The mice were randomized to receive rimonabant or placebo while maintained on the highly palatable diet. The authors asked: Would rimonabant help the mice lose weight even when they could eat as much delicious fatty food as they wanted?
Figure 1 Blocking CB1 may prevent weight gain
Δ9-THC activates the cannabinoid receptor (CB1), stimulating appetite and leading to weight gain in mice (left). When the same receptor is blocked, appetite is controlled (right).
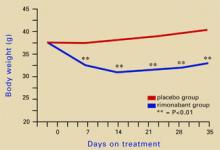
Source: Illustration for CURRENT PSYCHIATRY by Marcia HartsockRimonabant induced a sustained body weight reduction of approximately 20% in the treatment group compared with the placebo group across 5 weeks (Figure 2). Estimated fat stores among the treatment group were depleted by slightly more than 50%.
The authors noted that the mice in the treatment group had decreased their food intake, but the decrease was not sufficient to explain the weight loss. They speculate that rimonabant could activate metabolic processes and decrease intake.
RIMONABANT’S ROLE IN PSYCHIATRY
Phase III human trials of rimonabant are under way for obesity as well as smoking cessation.5 In uncontrolled studies, rimonabant has been shown to help people avoid weight gain while quitting smoking.5
If rimonabant shows effectiveness in controlled trials and is safe in humans, it could be most valuable. Obesity in industrial countries is epidemic and causes serious secondary morbidity, including diabetes, arthritis, and hypertension. Rimonabant, if approved by the FDA, could reach the market by early 2006.6
It is unknown whether rimonabant’s metabolic effects could offset those of many psychotropics. As psychiatrists, we often must stop an effective antipsychotic or antidepressant because it is causing significant weight gain. A treatment that would prevent medication-induced weight gain could improve patient compliance and, ultimately, outcomes.
MANAGING SCHIZOPHRENIA
Some evidence also suggests that rimonabant may offer additional benefits for patients with schizophrenia beyond weight reduction or smoking cessation.
Figure 2 Rimonabant’s effects on weight in mice on a high-fat diet
Source: Adapted from reference 4.Leweke et al found increased endogenous cannabinoids in the CSF of patients with schizophrenia, suggesting that a cannabinoid signaling imbalance may contribute to the disorder’s pathogenesis.7 However, 72 patients with schizophrenia or schizoaffective disorder who took rimonabant for 6 weeks showed no improvement compared with a placebo group.8