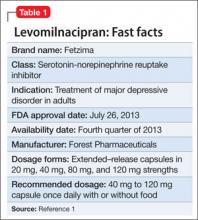
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3