according to data from an ongoing, nationally representative survey.
Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
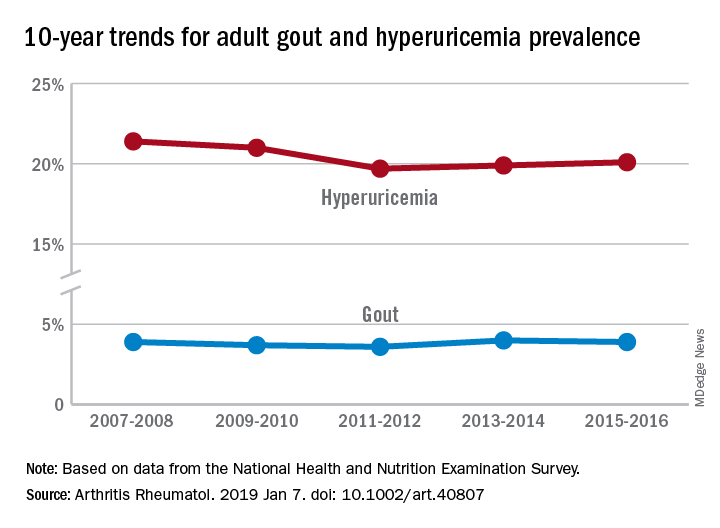
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.