Audio

Top Allergens Causing Contact Dermatitis
Dr. Mowad discusses the top allergens affecting patients and the diagnosis of allergic contact dermatitis.
From the Department of Allergy, Instituto de Investigación Sanitaria Princesa, Hospital Universitario de la Princesa, Madrid, Spain.
The authors report no conflict of interest.
Correspondence: Francisco Vega, MD, Hospital Universitario de la Princesa, Diego de León St 62, Madrid 28006, Spain (fvega13@hotmail.com).

Concomitant allergic reactions to multiple drugs are uncommon. We report the case of a 66-year-old woman who presented with concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. It is notable that one of the reactions was caused by oral nystatin, which generally is not considered to be allergenic due to its poor intestinal absorption. Diagnoses were confirmed on patch testing with histologic examination along with oral challenge testing. We also used challenge testing to rule out cross-reactivity among nystatin and other macrolide drugs, both antifungals and antibiotics.
Practice Points
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.
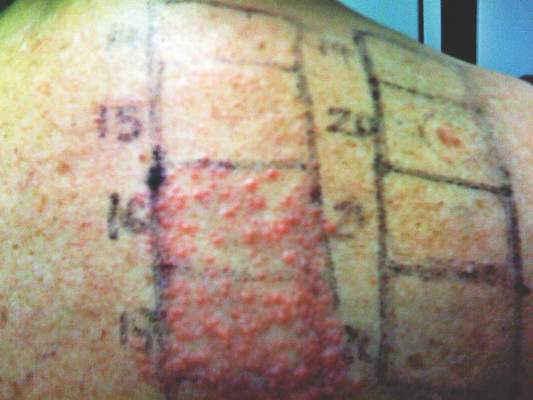
Patch test results at 96 hours for nystatin 2% in petrolatum (patch 14), nystatin 10% in petrolatum (patch 15), nystatin 30,000 IU in polyethylene glycol (patch 16), nystatin 90,000 IU in polyethylene glycol (patch 17), cinnamic aldehyde 1% in petrolatum (patch 19), paraben mix 16% in petrolatum (patch 20), petrolatum (patch 21), and polyethylene glycol (patch 22).
Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.

Dr. Mowad discusses the top allergens affecting patients and the diagnosis of allergic contact dermatitis.

The term latex allergy refers to a hypersensitivity to products containing natural rubber latex. Individuals with true latex allergy have...
