Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
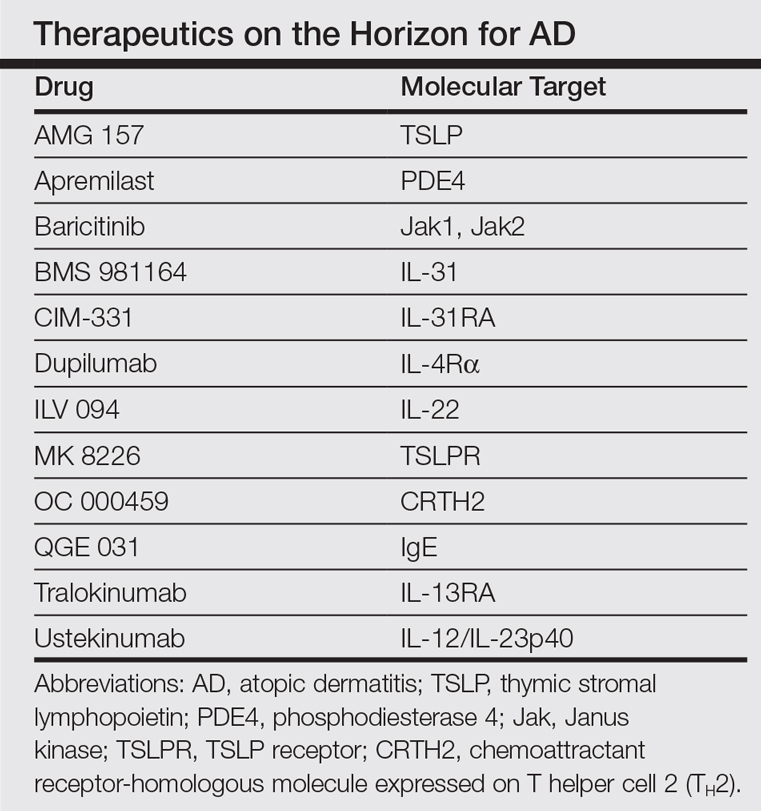
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.