To the Editor:
Mucous membrane pemphigoid (MMP) is an autoimmune blistering disorder that causes subepithelial damage and scarring of mucosal surfaces with or without skin involvement.1 The clinical presentation is highly variable. The oropharynx is the most common site of initial presentation, followed by ocular, nasopharyngeal, anogenital, skin, laryngeal, and esophageal involvement.2 Patients often present to a variety of specialists depending on initial symptoms, and due to the diverse clinical manifestations, MMP often is misdiagnosed. Our patient presented an even greater challenge because the disease progressed to tracheal and bronchial involvement.
A 37-year-old man presented to his primary care physician with a chief concern of a sore throat and oral ulcers. The patient was treated with a course of antibiotics followed by a nystatin oral solution. He continued to develop ulcerative lesions on the soft palate, posterior pharynx, and nasal mucosae. He sought treatment from 2 otolaryngologists (ENTs) and a gastroenterologist, and continued to be treated with multiple oral antibiotics, fluconazole, and topical nystatin. Despite treatment, the patient developed pansinusitis and laryngitis and presented to the ENT department at our institution with severe hoarseness and dyspnea on exertion. Examination by the ENT department revealed ulcerative lesions of the nares with stenosis and ulcers along the soft palate. Videolaryngostroboscopy showed remarkable supraglottic edema with thick endolaryngeal mucus. The patient worked as a funeral director and had notable formaldehyde exposure. He also hunted wild game and performed taxidermy regularly.
The patient was admitted and treated with intravenous dexamethasone for a compromised airway. Subsequently, he was taken to the operating room and had biopsies performed of the posterior pharynx. Given his exposure history, the infectious disease department was consulted and he was evaluated for multiple viral, bacterial, and fungal suspects including leishmania and tularemia. Age-appropriate screening, physical examination, and review of systems were negative for an underlying neoplasm. Histopathologic examination revealed a subepithelial vesicular mucositis with a mixed infiltrate of lymphocytes and histiocytes. Direct immunofluorescence microscopy demonstrated strong linear fluorescence along the epithelial-subepithelial junction with IgG and C3. Based on these findings, the diagnosis of MMP was made.
Further testing for bullous pemphigoid antigen 1 (BP230) and bullous pemphigoid antigen 2 (BP180) were negative. On one occasion the patient tested positive for anti-BP230 IgG, but it was at a level judged to be insignificant (7.5 [reference range, <9]). The patient also was negative for autoantibodies against desmoglein 1 and 3. Indirect immunofluorescence using rat bladder epithelium was not performed.
The patient was started on methotrexate and oral prednisone by the rheumatology department, but after 1 week, he presented in respiratory distress and was taken for an emergency tracheostomy. The patient eventually was referred to the dermatology department where methotrexate was discontinued and the patient was started on titrating doses of prednisone and mycophenolate mofetil. Eight weeks later, the patient became completely aphonic and was taken by ENT for dilation of the supraglottic, glottic, and subglottic stenosis with mucosal triamcinolone injections. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily was initiated in addition to mycophenolate mofetil 3 g and prednisone 80 mg, but again the patient developed near-complete tracheal stenosis just proximal to the tracheostomy entry site. At 16 weeks, balloon dilation was repeated with dexamethasone injections and topical mitomycin C. Subsequently, the patient regained some use of his voice. Although the next several laryngoscopes showed improvement in the patient’s epiglottis and glottis, the trachea continued to require debridement and dilation.
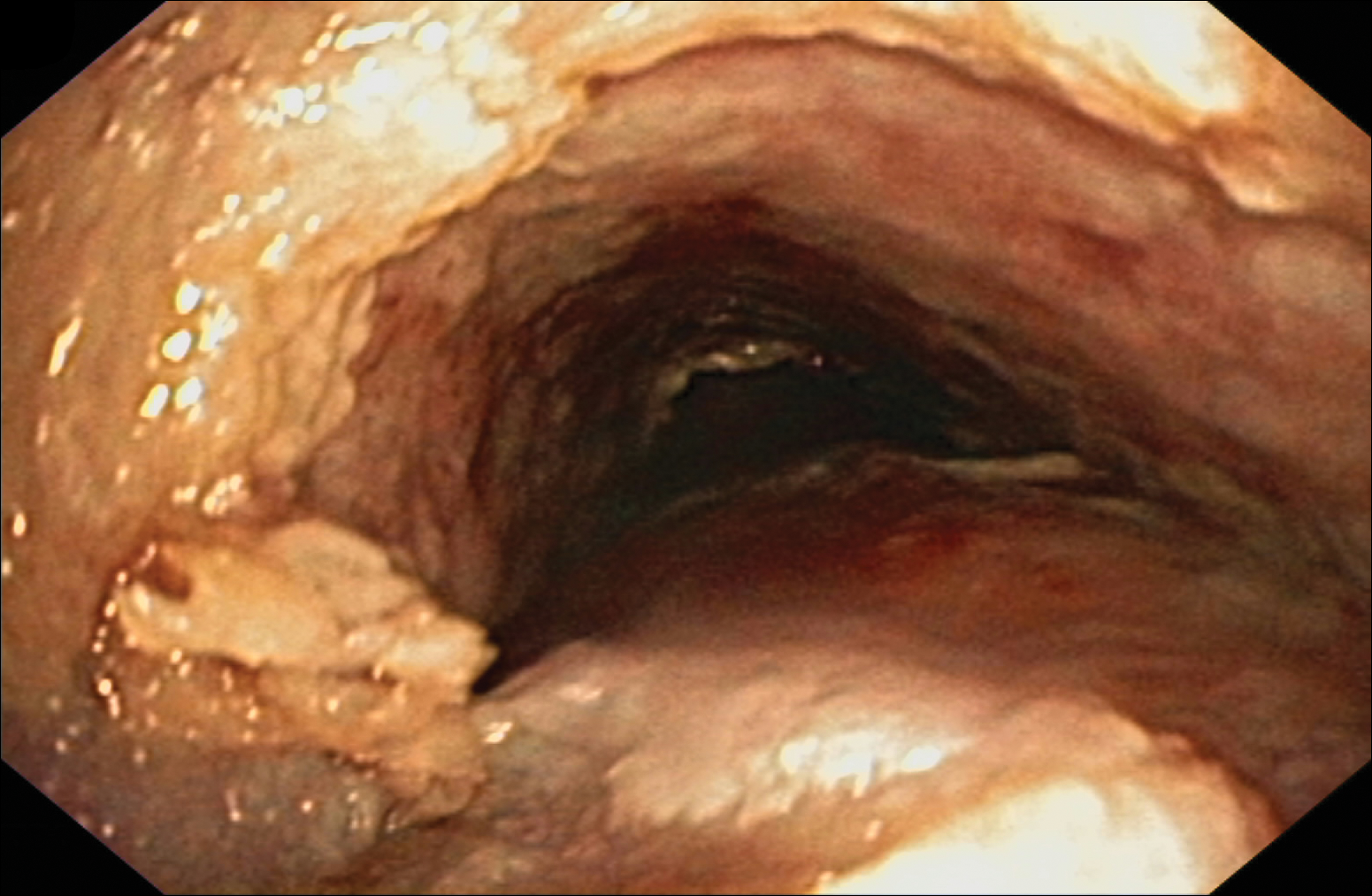
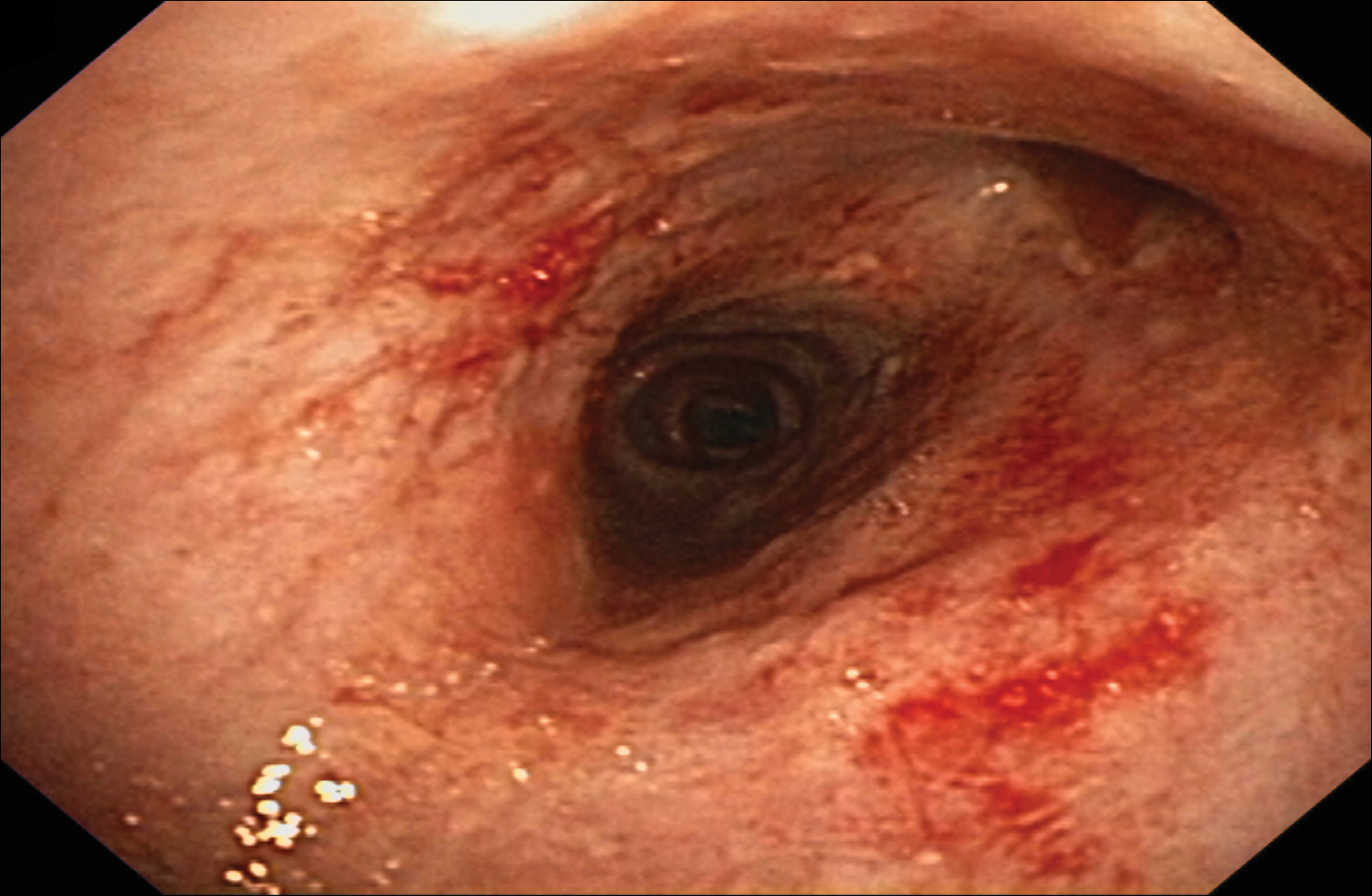
Despite maximal medical therapy and surgical interventions, the patient had little improvement in his voice and large clots of blood obstructed his tracheostomy daily. He was unable to sleep in his preferred position on the stomach (prone) due to dyspnea but had less distress sleeping on his back (supine). The patient was referred to the pulmonology department for an endotracheobronchoscopy to further evaluate the airway. It was discovered that the mucosa of the trachea from the level of the tracheostomy to the carina was friable with active erosions and thick bloody secretions (Figure 1). Lesions extended as far as the scope was able to visualize to the left upper lobe takeoff and the right mainstem bronchus (Figure 2). Biopsies of the carinal mucosa showed 3+/3+ linear fluorescence with IgG along the dermoepidermal junction. Salt-split studies were performed, but because the specimen was fragmented, it was not possible to assess if the fluorescence was present at the floor or at the roof of the split.
Given the severity of disease and failure to respond to other aggressive immunosuppressive therapies as well as having been with a tracheostomy for 22 months, the patient was started on 2 doses of intravenous rituximab 1 g 2 weeks apart along with trimethoprim-sulfamethoxazole (3 times weekly) for pneumocystis pneumonia prophylaxis. No complications were observed during infusions. After 2 rituximab infusions, he was weaned off of prednisone and a repeat bronchoscopy showed no airway ulcers beyond the distal trachea or endobronchial obstruction. However, the subglottic space and area above the tracheostomy showed remarkable stenosis with a cobblestone pattern and granulation tissue with continued narrowing of the subglottic area. The ENT performed further dilation and after 34 months, the tracheostomy was removed and a T-tube was placed. The patient required cleaning out of the T-tube approximately every 3 months, and after 2 years the original T-tube was replaced with a new one. At the time of this report, the ENT recommended removing the T-tube, but the patient was reluctant to do so; therefore, a second T-tube replacement is planned. He continues to do well without relapse and has been off all medical therapy for nearly 4 years.
Mucous membrane pemphigoid is an acquired autoimmune subepithelial blistering disease that predominantly affects mucous membranes with or without skin involvement. This condition has been referred to as cicatricial pemphigoid, oral pemphigoid, and ocular cicatricial pemphigoid, among other names. It is characterized by linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone. According to the international consensus on MMP, the target antigens identified in the epithelial basement membrane zone include bullous pemphigoid antigen 1 (BP230), bullous pemphigoid antigen 2 (BP180), laminin 5 (α3, β3, γ2 chains), laminin 6 (α3 chain), type VII collagen, and integrin β4 subunit.3 Not all patients with MMP will have circulating autoantibodies to the above components, and although our patient did have detectable anti-BP230 IgG, it was not considered clinically significant. Furthermore, the type of autoantibody does not impact decisions regarding therapy selection.3