Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
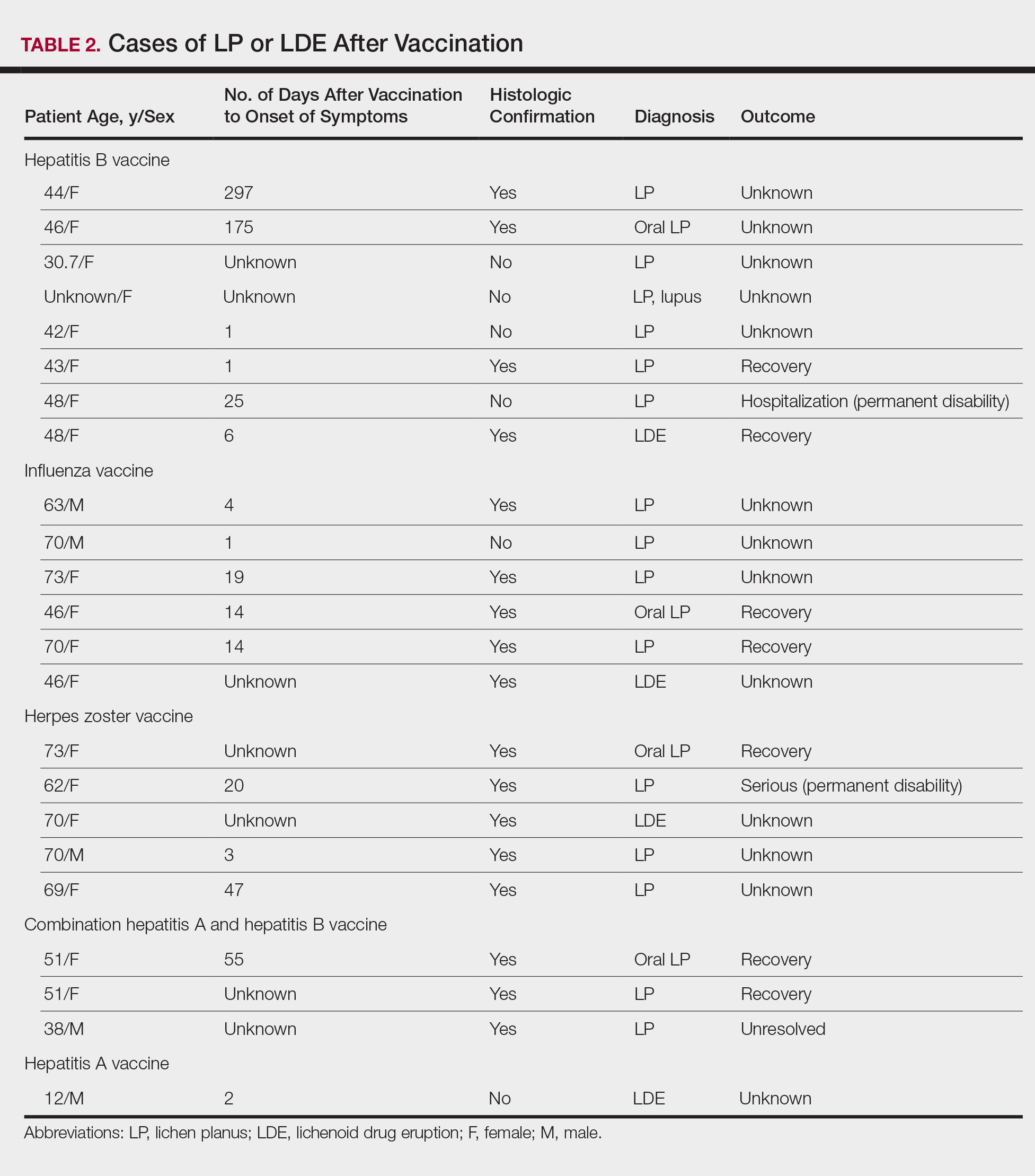
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
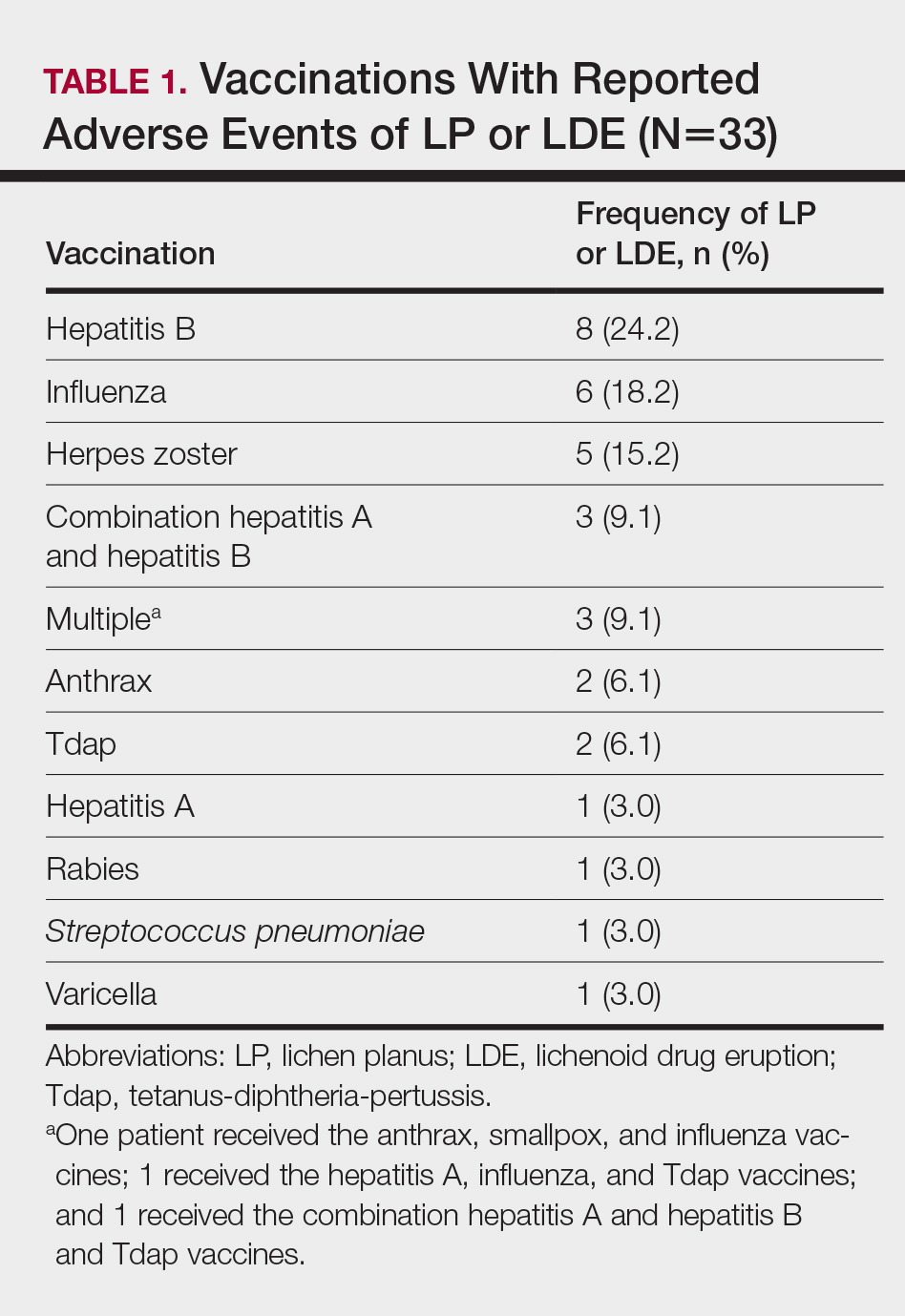
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. Of 5 cases of AEs associated with herpes zoster vaccination, 1 AE resulted in permanent disability.