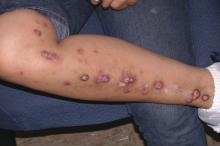
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Recent success in the use of (PN) suggests that safer and more effective systemic therapies that provide relief and management of this debilitating chronic skin condition are possible, reported Azam A. Qureshi, BA, of the department of dermatology, George Washington University, Washington, and his coauthors.
Their report was published in the Journal of the American Academy of Dermatology.
They conducted a systematic review of 35 clinical studies of different therapies for PN, published between Jan. 1, 1990, and March 22, 2018, using PubMed and Scopus databases. Their goal was twofold: to provide a summary of current evidence-based therapies and their corresponding level of evidence ratings, and to help researchers “identify gaps in PN treatment development and study.”
From 706 studies, the authors selected 35 clinical studies of treatment strategies for PN, for which the pathogenesis is virtually unknown, they noted. The studies included 15 prospective cohort studies, 11 retrospective reviews, 8 randomized controlled trials (with 10-127 patients), and 1 case series. Studies that failed to report treatment outcomes and those with fewer than five patients were excluded from the review.
Treatment modalities included topical agents, phototherapy and photochemotherapy, thalidomide, systemic immunomodulatory drugs, antiepileptics and antidepressants, and emerging treatment approaches. Many of the treatments evaluated in the review were found to offer limited promise for clinical application as a result of low efficacy or frequent side effects. The authors attributed the overall lack of success with treatments to the “heterogeneous nature of the etiology of chronic pruritus.”
Thalidomide was found to have limited use given its poor safety profile; lenalidomide achieved better outcomes, but treatment in one case was stopped because of possible drug-induced neuropathy or myopathy, they wrote. The systemic immunomodulatory agents methotrexate and cyclosporine exhibited successful treatment but also were found to have poor safety profiles. Greater promise was seen with antiepileptics and antidepressants, which were associated with fewer side effects. “Antidepressants, such as mirtazapine and amitriptyline, and antiepileptics, such as gabapentin and pregabalin, can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” senior author Adam Friedman, MD, professor of dermatology at George Washington University, said in an interview.
Among the most positive outcomes achieved were those in which promising newer treatments, including targeting IL-31 signaling and opioid receptor modulation, were used. In particular, the authors cited nemolizumab, an IL-31 receptor A antagonist, which is currently in a phase 2 trial of PN. Beneficial effects of extended release nalbuphine, an opioid k-receptor agonist and mu-receptor antagonist, were found in an unpublished multicenter, double-blind randomized controlled trial, with no serious adverse effects attributed to treatment.
Among the emerging treatments are the neurokinin-1 receptor antagonists, which include serlopitant, which showed beneficial effects compared with placebo in an 8-week randomized controlled study, they wrote. The neurokinin-1 receptor “is a target of substance P, a mediator of itch and a probable pathogenic agent in PN,” they wrote. “Binding by these agents likely disrupts substance P signaling, thereby halting PN pathogenesis,” they added.
“Antidepressants such as mirtazapine and amitriptyline and antiepileptics such as gabapentin and pregabalin can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” Dr. Friedman said in the interview.
Mr. Qureshi had no relevant financial disclosures. Dr. Friedman has received honoraria for participation in advisory boards for Menlo Therapeutics, and serves as an investigator for Kiniksa Pharmaceuticals. A third author received honoraria for participation in advisory boards for Menlo, and several other companies, and received grant/research support as an investigator from Menlo, Kiniksa, and several other companies.
SOURCE: Qureshi AA et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.020.