This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
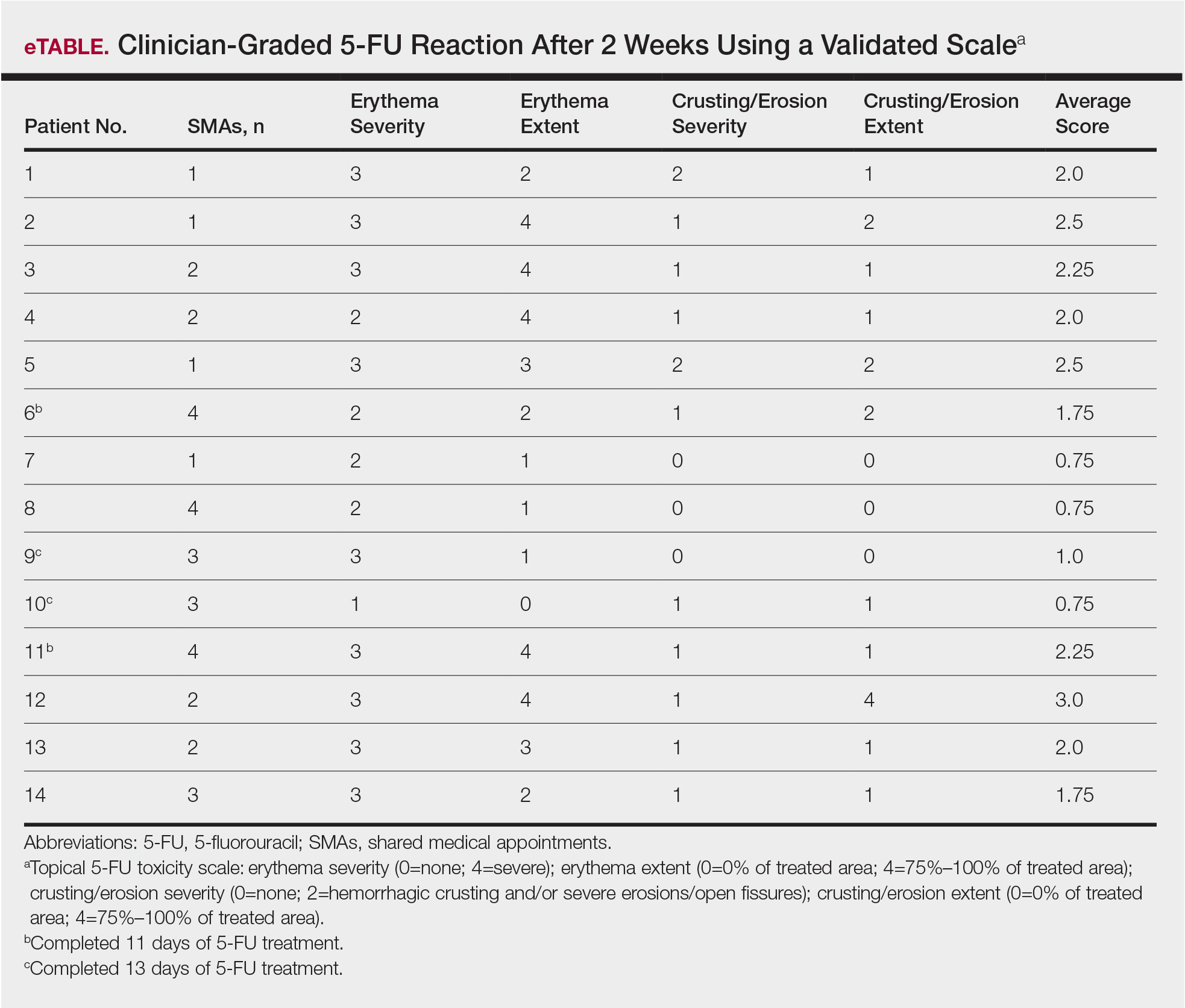
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.