Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
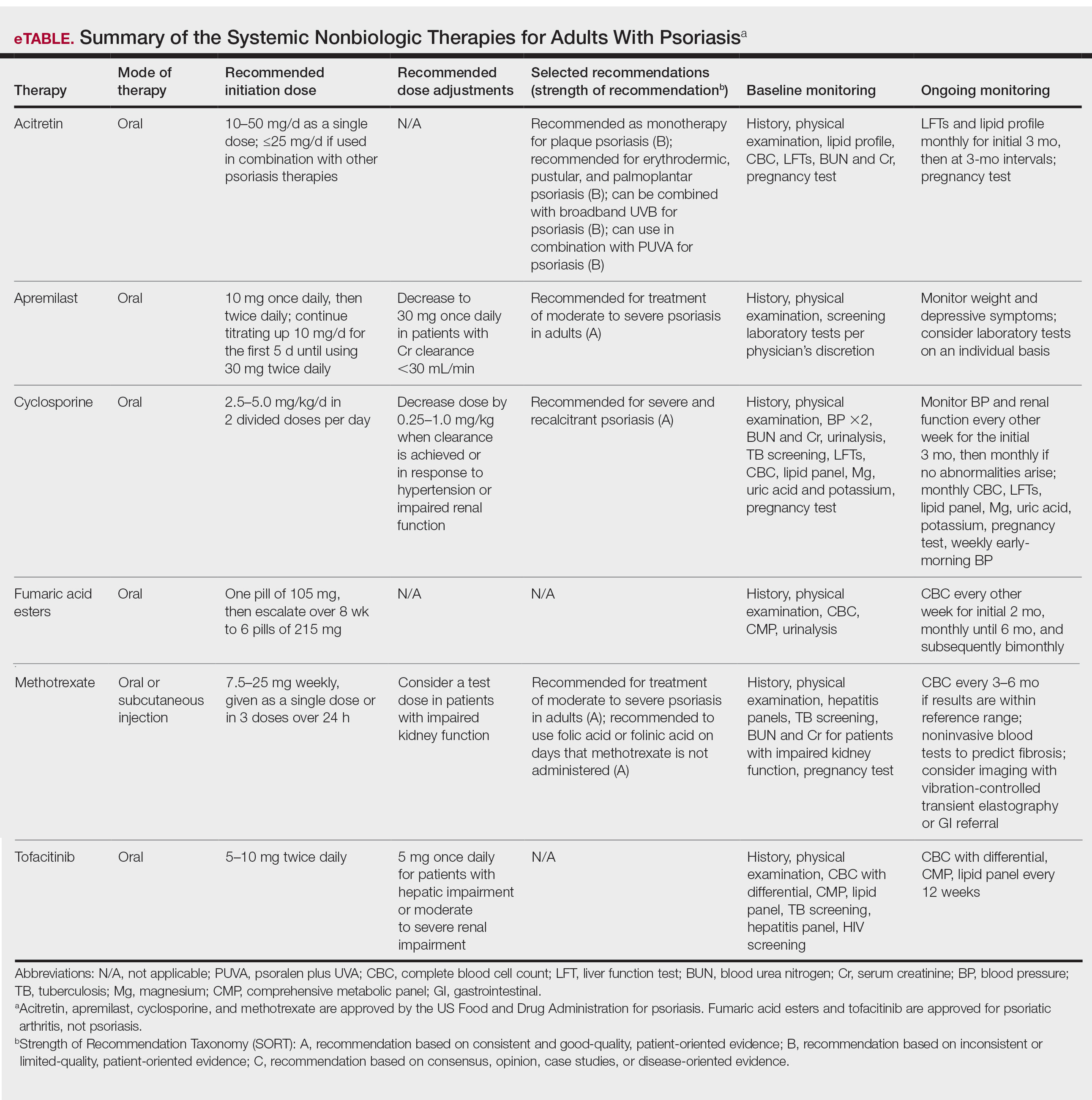
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7