Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
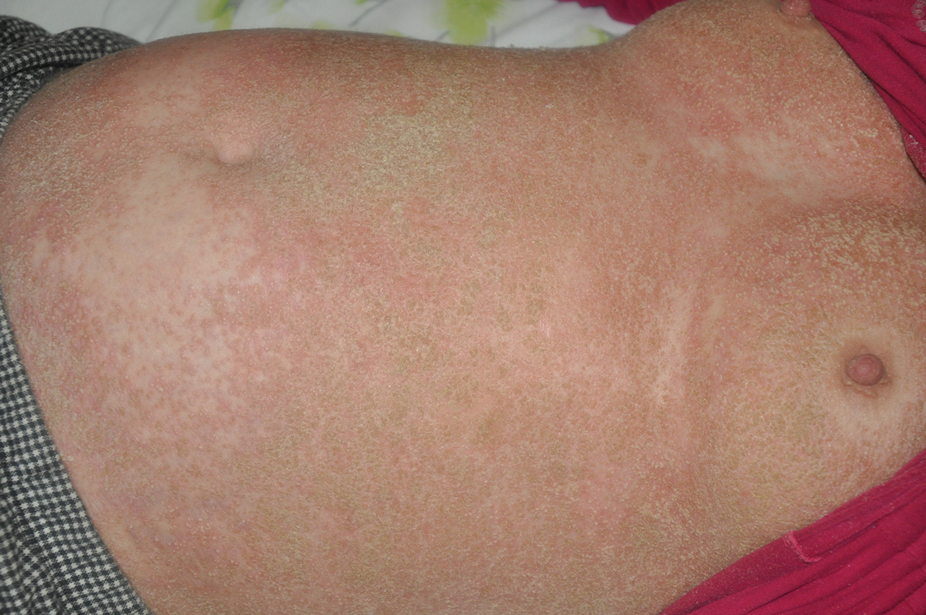
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.
Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.