To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
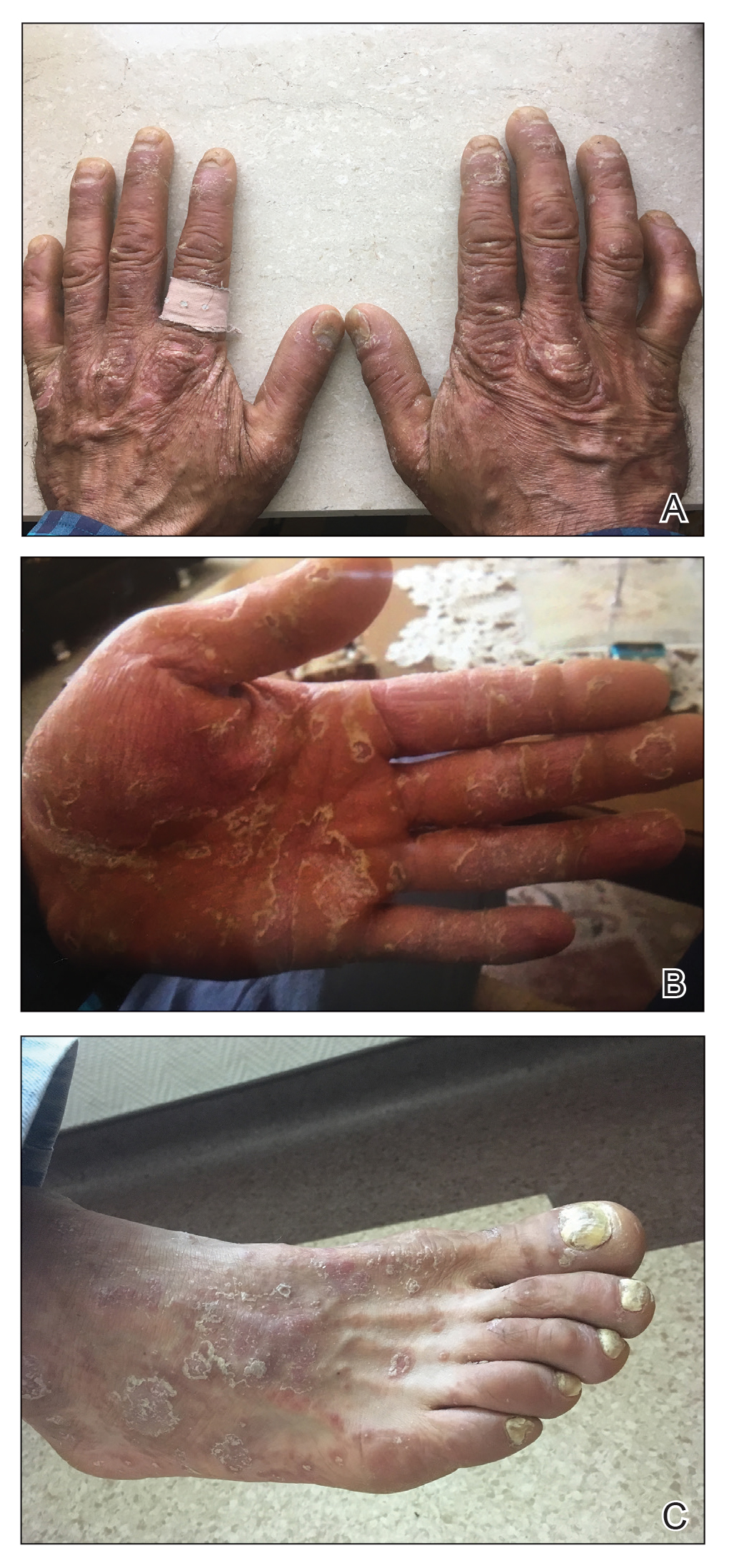
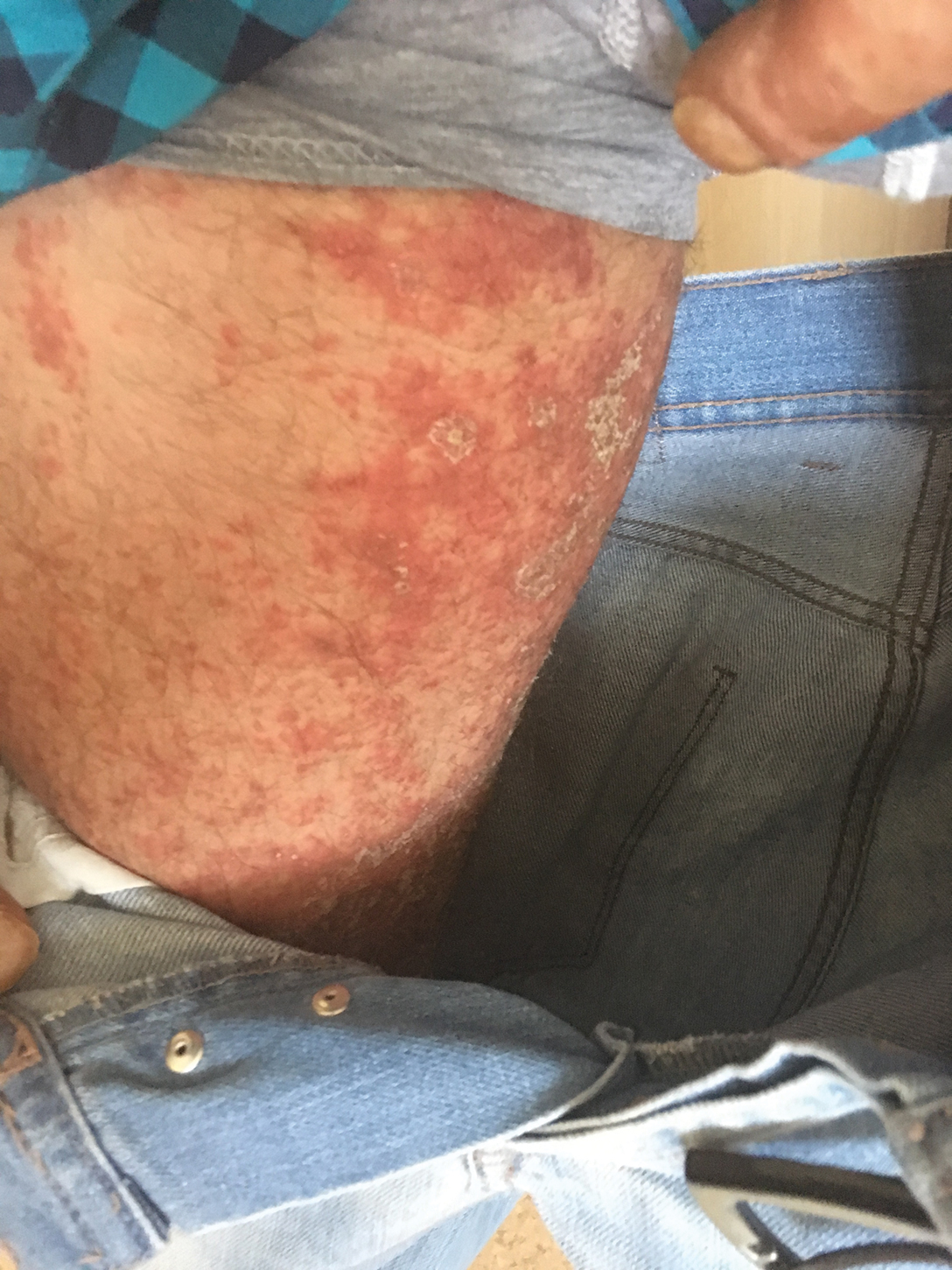
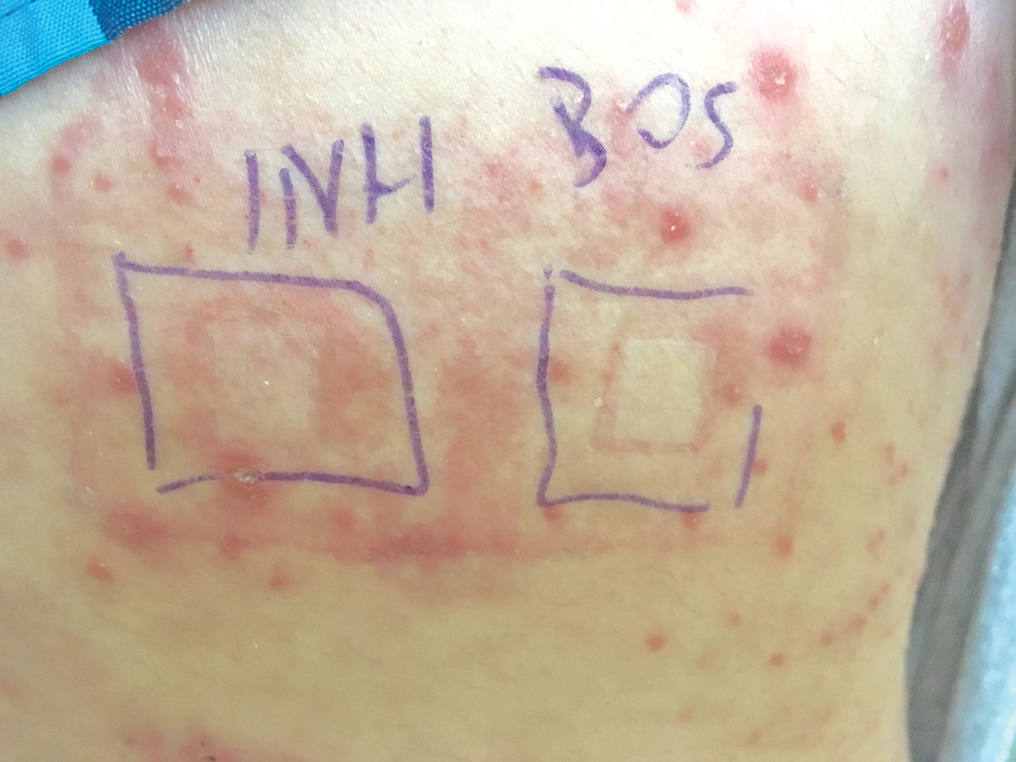
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.