Smallpox is caused by the variola virus and is capable of causing serious complications. The disease is highly contagious and may present in several forms. The variola major form has a case fatality rate of about 30%.1 The smallpox vaccine was the first immunization used in modern medicine. The first inoculation was given in 1796 by Edward Jenner, MD, who immunized an 8-year-old boy with material taken from cowpox. The inoculation was shown to protect the child from smallpox when he was inoculated 6 weeks later with material that was taken from a smallpox pustule. Since then, mass immunization against smallpox has become a well-established method of fighting the disease. Immunization is based on epidermal inoculation of live vaccinia virus, another poxvirus that provokes immunization against smallpox but does not have the ability to cause the disease. The inoculation method includes putting a droplet of vaccine on the skin and then superficially poking the skin sufficiently to cause minimal blood drops. Disease protection following immunization lasts about 10 years.2
Because humans are the only natural host of the variola virus, the World Health Organization proclaimed smallpox the first disease to be eradicated worldwide. This was accomplished in 1977, when the last naturally occurring case in the world occurred in Somalia. The eradication of smallpox made it possible to stop the use of the vaccine without the threat of natural recurrence of the disease. In many places around the world, immunization was stopped before 1977 because many years had gone by without any documented cases of smallpox. In the United States, the last case was documented in 1949, and routine smallpox immunization was discontinued in 1972. In Israel, it was discontinued in 1980.
Since the events of September 11, 2001, there have been major concerns about the possibility of bioterrorism using the variola virus, which had been kept in laboratories and could be used as an unconventional weapon by countries or militant groups. Because many individuals are not immunized against smallpox, either naturally or artificially, the potential of a major epidemic occurring is significant.3
Because routine immunization against smallpox was discontinued in the United States and Israel, entire populations are vulnerable to the disease. The possibility that Israel will be involved in war became stronger when the United States and its allies targeted Iraq as a country that develops weapons of mass destruction. The use of smallpox as a biological weapon is a major possibility.4
To prepare against smallpox, the Israeli authorities have decided on a 2-step plan. In the first step, emergency personnel are to be vaccinated. These include medical and paramedical personnel, as well as people in other emergency services. The second stage of the plan is to immunize the entire population of Israel if one case of smallpox is diagnosed.
The immunization of healthcare workers in the first stage has 2 purposes. First, medical and other emergency personnel are expected to deal with infected or potentially infected people and thus must be protected against the disease. Second, in case of an epidemic, serious life-threatening disease could be aborted by variola immunoglobulin. All those immunized prior to a smallpox epidemic are the potential donors of human gamma globulin against the disease.
Immunization of medical personnel in Israel started in September 2002. The immunization was voluntary and thus included only a portion of the potential recipients. I was immunized on September 24, 2002, at my workplace. Assuming that many practitioners do not have experience with the vaccine, I decided to keep a diary and take pictures of the changes at the site of the inoculation.
Case Report
On day 1, 3 drops of dried blood (1 mm each) appeared. There was no itching or redness. Days 2 and 3 were the same as day 1, except that the puncture spots became black. A round, red, 4-mm papule appeared on day 4. There was some itching, and the black puncture spots at the area of inoculation remained the same. The papule became a pustule 3 to 4 mm in diameter surrounded by a 2- to 3-mm rim of red papule on day 5, and itching intensified. On day 6, the pustule became 5 to 6 mm, and the red rim around it became 2 to 3 mm. The area was not painful, except when touched. Itching became more obvious, and there was some tenderness of the axillary lymph nodes, with minimal enlargement of the glands. The pustule became 6 to 7 mm, and the red rim became 3 to 4 mm, with an additional 1- to 2-mm pink rim by day 7. Headache was present.
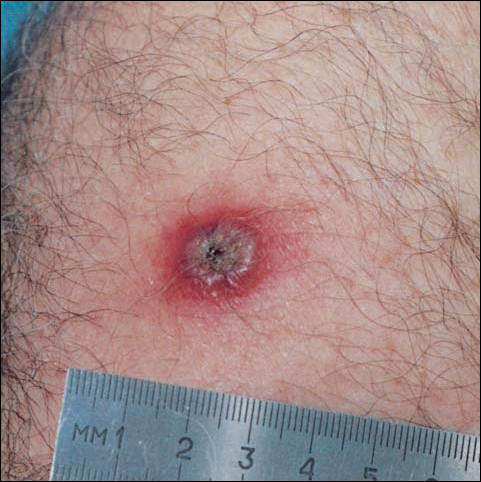
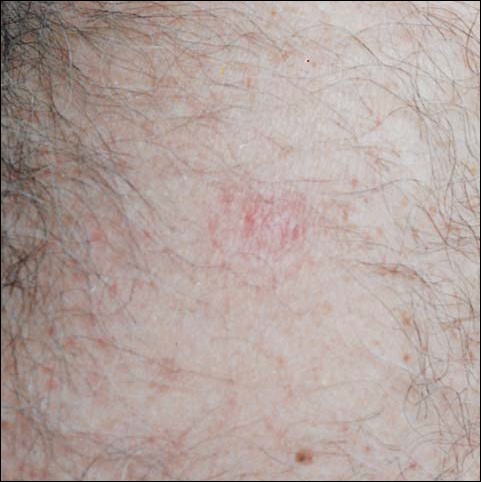
By day 8, the pustule was 10 mm, and the 3 dark 1-mm original puncture spots were still present inside the pustular area. The pustule felt hard when touched. The red rim was 2 to 3 mm, with an additional induration of skin 10 mm in diameter around the rim. The area was somewhat itchy, and headache was present. There was no change on day 9 except that the dark spots within the dry pustule were more obvious, increasing to 2 mm each. The size of the pustule was still the same on day 10, although it was less elevated. Central umbilication was obvious. On day 11, the pustule became dryer, and the black spots inside were 2 to 3 mm. Redness around the pustule was still 2 to 3 mm and was more intense after showering. Some induration of skin remained around the pustule and red area, and it was mildly painful when touched. Itching was lessened, and there was no pain at the axillary lymph nodes (Figure 1). The dry crust began to peel on day 12. The central area was confluent black, and the red rim was 2 to 3 mm, with some itching. On day 13, the crust had no yellow color of dried pus, and the roof of the crust had peeled off completely. The exposed base of the crust was dark black and hard. One to 2 mm of the red rim was still present, with less surrounding induration. There was mild itching but no pain or discharge. Minimal change was seen on day 14, except that the lesion began to shrink slightly.
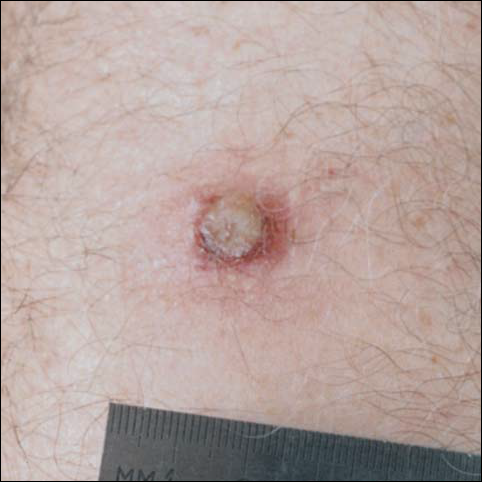
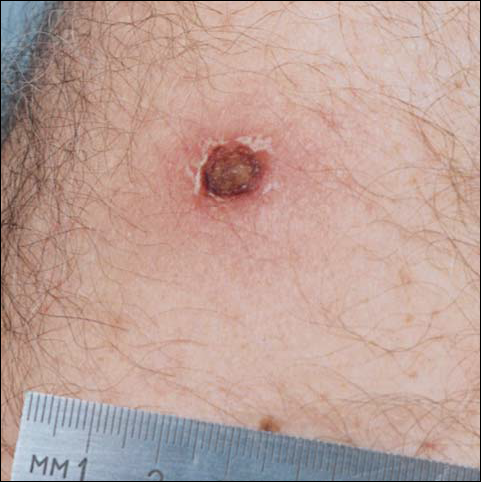
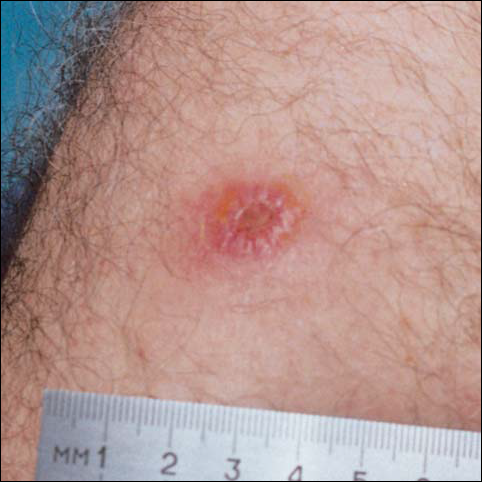
There was no pain on day 15, but the borders of the black crust began to separate from the red rim, and the red rim decreased to 1 to 2 mm. Itching became more intense, and the crust bled slightly at its border when touched. An adhesive bandage was put on the area. When the bandage was removed on day 16, it looked as if there was liquefaction of the crust, which had a yellowish color (Figure 2). The area was covered by gauze to enable air drying. On days 17 and 18, the crust was dry and brown, with no bleeding. It appeared smaller than the day before, the red rim had become pink, and there was mild itching (Figure 3). The skin above the pink rim began to peel on day 19, and there was no change in the scab. On one of the borders, there was a small discharge of serum. The scab fell off after showering (Figure 4). An ulcer 1- to 2-mm deep was obvious at the spot; the rim around the ulcer was red. The ulcer was smaller on day 20, and the rim around the ulcer was pink and still elevated above the surrounding normal skin. There was no discharge. On day 21, the base of the ulcer was dry and brown.
On day 22, the skin around the brown area was dry and pink, and the rim was 1 to 2 mm. Minimal changes were noted from days 23 to 27, and there was no itching. There was still no itching on day 28, and the red rim had disappeared. The central brown area peeled off, leaving a 4-mm pinkish red macule.
From days 29 to 75, the area gradually became pink. The skin looked thin and wrinkled, and there was no umbilication or elevation above the surrounding skin (Figure 5). No scar or induration was present, and symptoms had subsided.
Comment
According to the US Centers for Disease Control and Prevention (CDC), if the smallpox vaccination is successful, a red and itchy bump will develop at the vaccine site in 3 to 4 days. In a week, the bump becomes a large blister, fills with pus, and begins to drain. During week 2, the blister begins to dry up, and a scab forms. The scab falls off during the third week, leaving a small scar.5 The described case follows the general description of the CDC. However, some differences should be mentioned. Itching at the site of vaccination was a major accompanying symptom. Swelling beyond the immediate site of skin reaction was obvious and lasted for more than a week. Axillary lymphadenopathy was noted. Discharge from the pustule was minimal. A scab was formed twice—once as the pus dried up and again after the first scab fell off and the drying of the wet base of the first scab dried up. At the end of the skin reaction, no scar remained.
Could the differences between the case described and the description by the CDC be explained by personal variation in skin reaction? Is the difference dependent on the skin care following the vaccination?
The instructions that were given to the Israeli recipients of the vaccine were to keep the area covered with loose gauze, change the gauze daily, not change regular skin washing habits, not touch the place of vaccination, and not put any topical preparation on the site. I followed the instructions except on day 15, when bleeding began as the scab started to peel off. At that point, a bandage was put over the area, thus limiting the ventilation and drying of the scab.
Complications following smallpox vaccination are relatively rare. Haim et al6 described an overall complication rate of 0.4 per 10,000 young army recruits. The complication rate was higher in primary recipients (those without previous exposure to the disease or vaccine).
Feery7 described an adverse skin reaction rate of 118 per 1 million recipients and a death rate of 1 to 5 per 1 million. The more serious skin reactions were eczema vaccinatum, progressive vaccinia, and neurologic and cardiac complications.
Monitoring the reaction to the vaccine is important in evaluating the intervention. Checking antibody titers postimmunization is possible. The micro–enzyme-linked immunosorbent assay technique was found to be more sensitive than plaque neutralization in finding a 4-fold increase in antibody titers.8 Postimmunization antibody checking is not done routinely, and it is not feasible when mass immunization is done over a short period as a crisis intervention.
As described, one of the reasons for the immunization of health workers in Israel is to build a pool of potential donors for variola immunoglobulin. The CDC description of a successful immunization includes the formation of a scar at the site of vaccination. Barghini9 has described an unusual reaction in an adult after revaccination against smallpox. In his case, there was a delay in the loss of the crust and a lack of cicatrix. In my case, crust appeared twice, and there is no remaining scar.