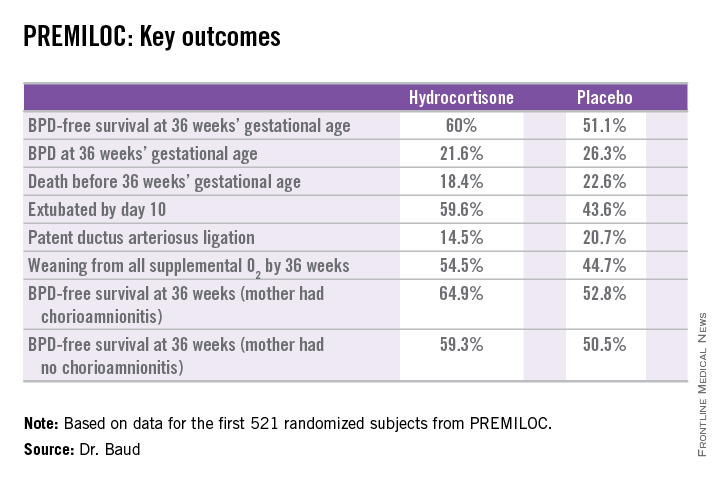
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.
He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.