Ms. Yates: Were there any final recommendations about informed consent?
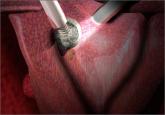
Dr. Iglesia: A lot of discussion, particularly on the second day, was in the area of labeling special controls and it would be labeling for a patient and practitioners or physician surgeons who are using the morcellator. To the extent that—and there have been some precedents I think in silicon breast and other devices—where both the patient and the physician have to sign off that they’re aware that morcellators may be used, that there’s a potential for dissemination of an occult malignancy or even dissemination of benign disease like leiomyomatosis and incomplete removal. There are risks of using the morcellator in terms of injury, just a whole checklist. But it’s interesting for the labeling, both the patient and the physician in this: One of the recommendations for special controls would be included.
Ms. Yates: And would that involve a black box warning?
Dr. Iglesia: I think there were several discussions about the black box warning; I’m not sure what the final discussion is. Some people believe that with the black box on an administrative level, it sends a signal and a reminder to everyone about the labeling. But labeling can be done without a black box and it can be done with a black box. I’m not 100% certain how that will ultimately be decided upon by the FDA.
Ms. Yates: What were your reactions to the hearing, apart from your role on the panel? Did you feel that adequate testimony was heard from all the parties that have a stake in the immediate and long-term fate of laparoscopic power morcellation?
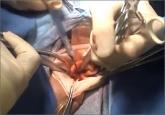
Dr. Iglesia: I think that the FDA did an excellent job in convening all the players, anybody who has interests in the stake I feel was represented--from industry and companies that make morcellators, companies that make containment bags, medical societies, ACOG, and AAGL gave testimony. AAGL’s testimony was very powerful, particularly by Dr. Jubilee Brown in mentioning that without the morcellator more women may be subjected to abdominal procedure, which in and of itself has some morbidity and mortality associated with that type of operation, and it was a nice study analysis. In terms of a decision tree what the potential harms would be without available morcellators to use, and I thought the MRI imaging that was done by the radiologist was also very interesting and discussed the limits of our ability to detect.
I also found some of the testimony to be extremely powerful from the patients, including that of Dr. Amy Reed and her family and the other women who presented. In some of the cases, we and several people on the panel, including myself, did wonder about the selection or the choice to use the morcellator in the first place in some cases, particularly in women who had uterine fibroids and they were postmenopausal. I think that that would be a particular case where you know go ahead and make an incision because there’s a potential higher index of suspicion for cancer in those kinds of cases.
Ms. Yates: Do you care to predict whether gynecologic surgeons will continue to use power morcellators after this controversy?
Dr. Iglesia: You know, and this was also discussed by Dr. Fisher and some of the officials from the FDA, that if anything this would be a call for innovation and improving products that could morcellate and contain at the same time. I know that we have some hysteroscopic morcellators that you can insert and there’s a vacuum and so things get kind of vacuumed up and whether or not we can develop something that has very little spill—obviously none at all would be key—and I do believe that at some point there will be some ingenuity and some improvements made to the current devices that will allow us to continue this is in our armamentarium.
What was interesting was that one of the questions that was addressed to the panel was, “When do you see that the benefits may outweigh the risks, in what population?” And leiomyosarcoma, which is just one of the occult malignancies—and there’s different types of sarcomas including endometrial stromal sarcomas and other endometrial cancer and malignancies. What’s interesting is that when you look at fibroids and even when you do a myomectomy it’s not necessarily just the power of morcellation, it’s just cutting through cancer or morcellating either vaginally or open. You’re doing an open myomectomy, just removal of the fibroid, and it turns out that that’s cancer. You know that is not a good prognosis to start with, but to spread it clearly is not good for the patient and makes a bad condition even worse.