given the dramatic increase in information available over the past decade.
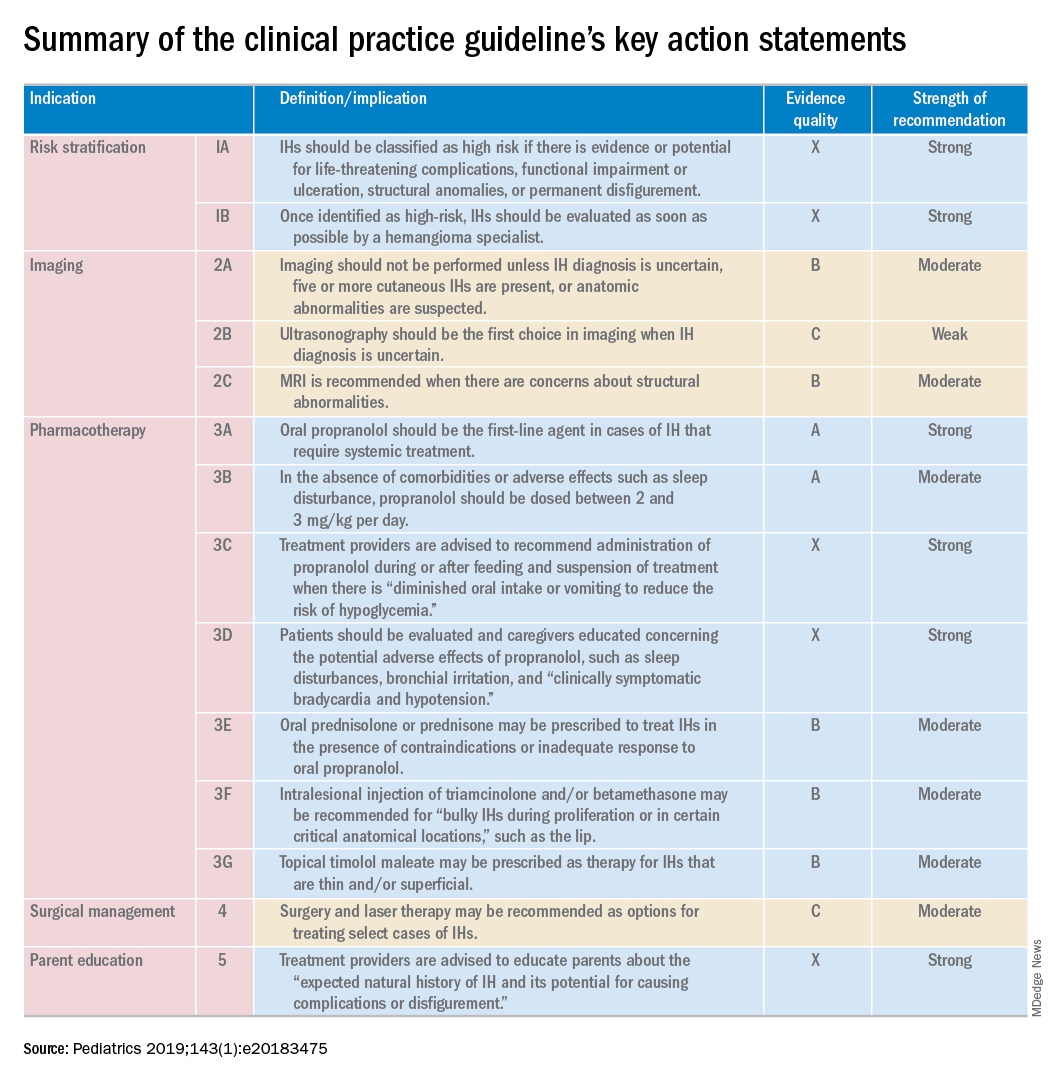
The aim in providing an evidence-based approach to evaluating, triaging, and managing IH cases is to arm primary care providers with the confidence needed to successfully treat high-risk cases, reported Daniel P. Krowchuk, MD, of the department of pediatrics and dermatology, Wake Forest University, Winston-Salem, N.C., and his associates who are members of the AAP subcommittee on the management of IHs.
With an occurrence rate of 4%-5%, IHs are the most common benign tumor presenting in childhood, especially occurring in girls, twins, preterm or low-birth-weight infants, and white neonates.
The AAP’s guideline “provides a framework for clinical decision-making” – it should not be considered a sole source of guidance. It also should not be used to replace clinical judgment or as a protocol for managing all patients with IHs, explained Dr. Krowchuk and his associates.
Clinicians, especially, are encouraged to consult promptly with a hemangioma specialist if they are not experienced in managing IHs.
According to one study cited by the authors, the mean age of examination by a dermatologist is 5 months, when most growth has already been completed. Lesions are first noticed, on average, at 2 weeks; 4 weeks has been recommended as the ideal time for professional consultation. It is important for clinicians to recognize the difficulty families are likely to face in obtaining an appointment, which makes caregiver and clinician advocacy on behalf of infants affected critical, urged Dr. Krowchuk and his colleagues. In cases or locations where hemangioma specialists are in short supply, telemedicine triage or photographic consultation is especially helpful.
Dr. Krowchuk and his associates noted several possible challenges in implementing this clinical practice guideline (CPG) published in Pediatrics. The growth of individual IHs is difficult to predict, especially in young infants, and there are no markers or imaging studies to correct this challenge. For this reason, they advised: “Prompt evaluation, either in-person or via photographs, is warranted for any infant reported by parents to have a changing birthmark during the first 2 months of life.”
Wide heterogeneity in terms of size, location, patterns, of distribution, and depth, when coupled with unpredictable growth, makes management of IHs unpredictable. Thus, there can be no one-size-fits-all treatment approach.
Further complicating implementation of the CPG is the long-held myth that IHs are benign and resolve spontaneously. While this may accurately describe the vast majority of outcomes, “ample evidence” demonstrates what can happen when family and/or caregivers yield to such “false reassurance.” According to Dr. Krowchuk and his associates, hemangioma specialists have seen their share of “examples of lost opportunities to intervene and prevent poor outcomes because of lack of or delayed referral.”
The paucity of data on high-risk cases in primary care and referral care settings should be the subject of future research, the authors noted. Scorings systems, such as the Hemangioma Severity Score, are growing in popularity as a triage tool, but more research is needed to demonstrate that primary care physicians are accurately interpreting findings and that high-risk cases are accurately identified to avoid over-referral to specialists.
Dr. Krowchuk and his colleagues did call attention to important evidence gaps that may be answered by research currently underway, or that may require further research in the future by asking the following questions: How safe is treatment with topical timolol in early infancy, and what proportion of patients can be observed without referral? For healthy infants 5 weeks or older, to what extent, if any, is cardiovascular monitoring for propranolol necessary? How should pediatricians be involved in beta-blocker management of infants and when should specialty reevaluation be made? What is the accuracy of primary care identification of high-risk IH cases using many of the parameters offered within this CPG? Are pediatric trainees being sufficiently trained in stratifying and managing IH risk?
One noteworthy barrier to improved management and outcomes noted by the authors is the “imprecision of current diagnostic codes.” At present, the existing coding in the International Classification of Diseases, 10th Revision does not include specific reference to IH but rather describes “hemangioma of the skin and subcutaneous tissues” and can include congenital as well as verrucous hemangioma. The codes also do not address the details characteristic of IHs or the higher risk aspects of IH, such as location or multifocality. Advocacy, in this instance, would be appropriate, advised Dr. Krowchuk and his associates.
In an interview, Dr. Krowchuk provided additional insight into what sets the AAP’s CPG apart from consensus statements published previously by European and Australasian expert groups. Although these might appear to be similar documents with analogous content at first glance, there are important differences, he said.
The consensus statements were based on expert opinion, while “the academy’s CPG was founded on an extensive review of the medical literature (1982-2017) regarding the potential benefits and harms of diagnostic modalities and pharmacologic and surgical treatments,” Dr. Krowchuk explained. The information that came out of this extensive review is what members of the subcommittee used to develop key action statements that pediatricians can use to evaluate and manage infants with IHs.
“The scope of the consensus statements was more limited, focusing primarily on the treatment of IH with propranolol. While the benefits of propranolol, its use and dosing, and potential adverse effects were addressed in depth in the academy’s CPG, the document went well beyond this,” he clarified.
The AAP also previously published a clinical report that provides a comprehensive evaluation of the pathogenesis, clinical features, and treatment of IH (Pediatrics. 2015 Oct. doi: 10.1542/peds.2015-2485).
There was no external funding for the CPG, and the authors said there were no potential conflicts of interest. Ilona J. Frieden, MD, is a member of the data monitoring safety board for Pfizer and the scientific advisory board for Venthera/Bridge Bio; Anthony J. Mancini, MD, said he has advisory board relationships with Verrica, Valeant, and Pfizer.
SOURCE: Krowchuk, DP et al. Pediatrics 2019;143(1):e20183475.