To the Editor:
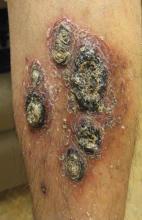
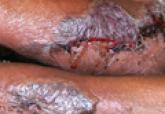
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.