Case Letter

Marjolin Ulcer in a Surgical Scar
Marjolin ulcers are malignancies arising in nonhealing cutaneous wounds.
Camilla Vassallo, MD, PhD; Andrea Carugno, MD; Federica Derlino, MD; Olga Ciocca, MD; Valeria Brazzelli, MD; Giovanni Borroni, MD
From the Department of Clinical-Surgical, Diagnostic, and Pediatric Science, Institute of Dermatology, Fondazione IRCCS PoliclinicoSan Matteo, University of Pavia, Italy.
The authors report no conflict of interest.
Correspondence: Camilla Vassallo, MD, PhD, Department of Clinical-Surgical, Diagnostic, and Pediatric Science, Institute of Dermatology, Fondazione IRCCS Policlinico San Matteo, Viale Camillo Golgi 19, 27100 Pavia, Italy (cvassallo@yahoo.com).

Classic Kaposi sarcoma (KS) usually is a localized and slowly progressing disease that mainly affects elderly patients; therefore, local treatment generally is recommended. In this study, we evaluated the clinical efficacy of intralesional vinblastine (VNB) for the treatment of classic KS in 6 participants with type 2 diabetes mellitus. Results indicated that intralesional VNB injections may be an effective alternative treatment of classic KS in diabetic patients.
Practice Points
Kaposi sarcoma (KS) is caused by infection with human herpesvirus 8, a DNA virus and member of the Gammaherpesvirinae subfamily.1,2 Cutaneous KS lesions generally appear as red to purple macules, plaques, and/or nodules that can become ulcerated, causing pain and bleeding. Lesions often are localized on the feet, which may impede walking and daily activities. Early diagnosis and management are important to avoid or minimize severe symptoms (eg, enlargement of lesions, ulceration with bleeding and pain). Injection therapy is recommended for the management of localized disease, while systemic therapy is appropriate for disseminated malignancy.3 There currently is no curative therapy available for KS; however, palliative therapy can avoid or reduce cosmetically unacceptable lesions and painful edema.
Vinblastine (VNB) is a natural vinca alkaloid whose primary cytotoxic effect is prevention of mitotic activity.4 However, antiangiogenetic activity has been demonstrated with low concentrations of VNB.5 The use of intralesional VNB injections for the management of oral KS in open-label studies4 using different doses and multiple courses has proven to be therapeutic.6-8 There are limited studies regarding the use of intralesional VNB injections in patients with cutaneous KS, particularly in those with associated type 2 diabetes mellitus.6-8 Thus, we evaluated the clinical efficacy of intralesional VNB injections in the treatment of KS lesions in diabetic patients with no visceral involvement.
Methods
This study was conducted in the department of dermatology from January 1, 2009, to December 31, 2011, and was approved by the institutional review board of the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy). Six patients with type 2 diabetes mellitus and histologically diagnosed cutaneous KS were enrolled in the study. Inclusion criteria included histological diagnosis of KS, negative human immunodeficiency virus testing, history of type 2 diabetes mellitus, more than 5 cutaneous KS lesions, and no visceral involvement (confirmed by colonoscopy, gastroscopy, lymph node and abdominal echography, and chest radiograph). Informed written consent was obtained from all participants.
Treated plaques and nodules measured more than 1 to 2 cm in diameter. Intralesional vinblastine injections were administered, not perilesional, with a standard concentration of 0.5 mg/mL per 1-cm2 lesion (maximum dose, 2 mg daily). All participants received 1 injection per nodule and a maximum of 2 injections per plaque. Monitoring of the target lesions was done via photography and mapping. Clinical evaluation of the treated lesions and assessment of side effects was conducted at 1-week and 1-, 3-, and 6-month follow-up. Complete response was defined as 95% to 100% remission of the target lesions, partial response was defined as 50% to 95% remission, and minimal response was defined as less than 50% remission, all at 3-month follow-up. No response was defined as no change or increase in size of the target lesions. Side effects including pain, hyperpigmentation, and ulcer formation were evaluated.
Results
A total of 6 participants (5 men, 1 woman; age range, 66–82 years) completed the study. Baseline demographics and clinical characteristics of the participants are reported in Table 1. Three participants were currently undergoing diabetic therapy with metformin, 2 were receiving insulin injections, and 1 was following a diabetic diet. None of the participants had internal KS. All 5 participants with nodular KS lesions previously underwent surgical excision. One participant also underwent radiotherapy with a good initial outcome, but lesions recurred at the same site within 1 year.
Complete response of nodular KS lesions and partial response of plaques to intralesional VNB injections was observed at 3-month follow-up in all treated lesions (Table 2). Five participants achieved a greater than 50% reduction (overall decrease in size and flattening of the plaque) of the KS plaque lesions. No participants showed minimal or no response.
At 1-week follow-up, shrinkage of nodular lesions was evident with necrotic crusts. Participant 4 who presented with plaques under the feet developed lymphatic serum exudation in the first 3 days following treatment on the legs. All the treated nodular lesions resolved completely within 3 months after treatment (Figures 1 and 2), though some new lesions appeared in new locations. All the treated plaques showed partial response to treatment after 1- to 3-month follow-up (Figure 3).
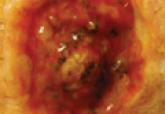
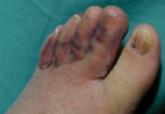
| Figure 1. An ulcerated bleeding nodular lesion of Kaposi sarcoma localized at the base of the first toe (a possible site of infection) in a diabetic patient (participant 1)(A). Three months after a 0.2-mg/mL intralesional vinblastine injection, the lesion had completely resolved (B). |
| Figure 2. A 2-cm nodular lesion of Kaposi sarcoma in the malleolar region of a diabetic patient (participant 2), a common site of venous ulceration in this patient population (A). One month after a 0.5 mg/mL intralesional vinblastine injection, complete response was noted without signs of ulceration (B). |
| Figure 3. A diffuse recurrent plaque of Kaposi sarcoma on the plantar region in participant 4 that had been treated with radiotherapy 2 years prior (A). Three months following a 3-mg/mL intralesional vinblastine injection, partial response was evident (B). |

Marjolin ulcers are malignancies arising in nonhealing cutaneous wounds.
Keratoacanthomas are rapidly growing, typically painless, cutaneous neoplasms that often develop on sun-exposed areas. They can occur...
Kaposi sarcoma (KS) is a malignant proliferation of endothelial cells within the skin. The clinical presentation is characterized by clusters of...
