IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
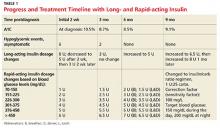
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>