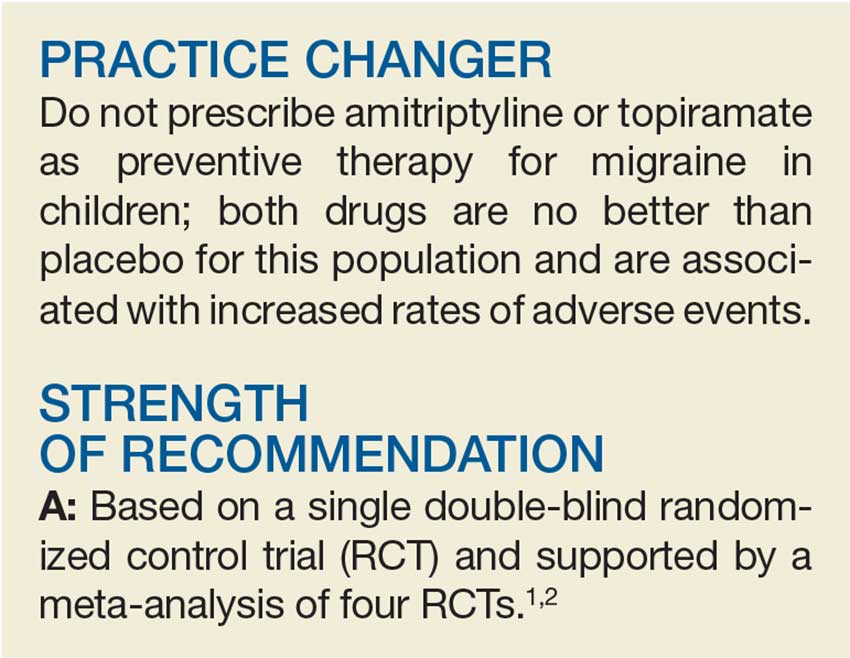
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...