Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
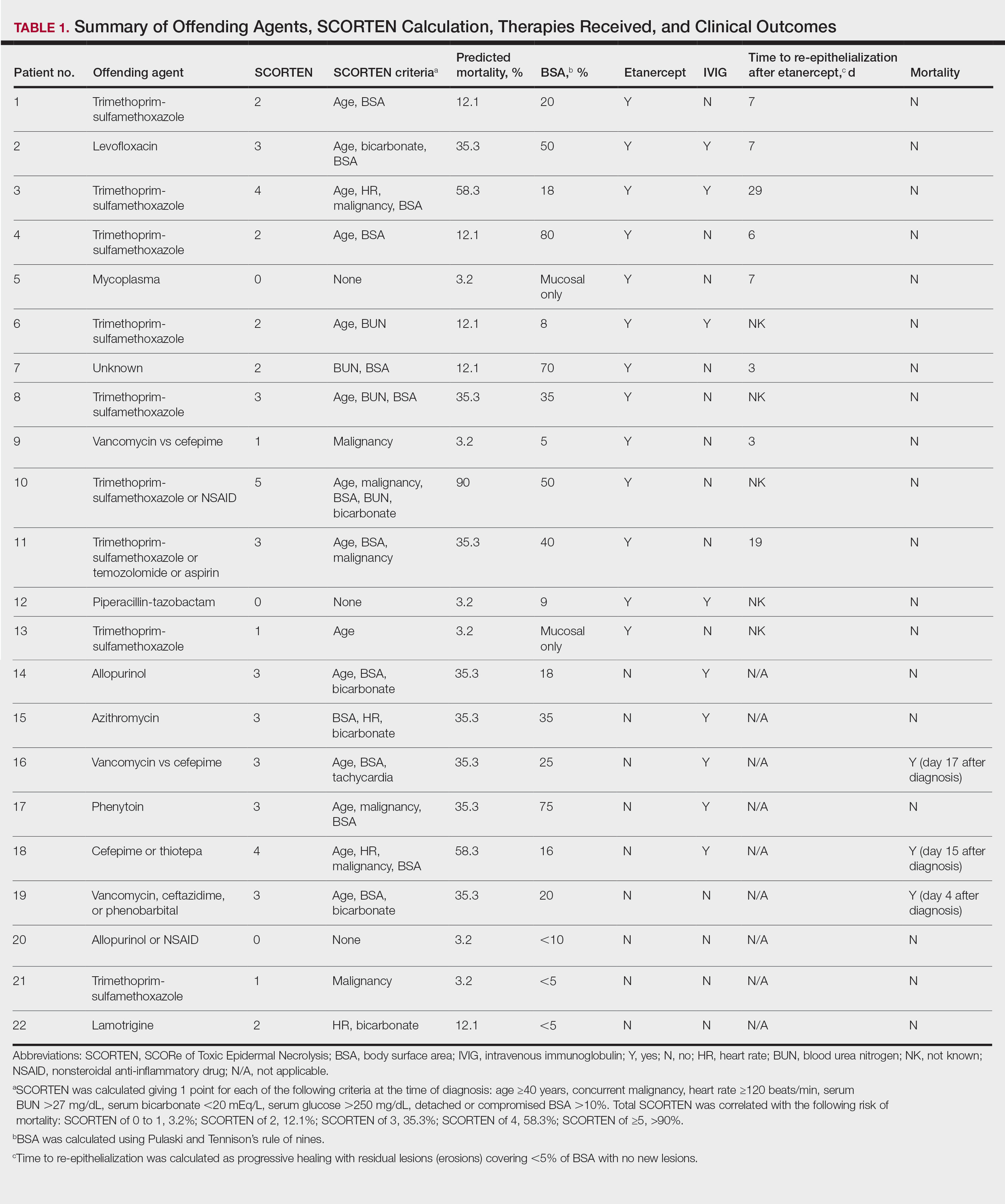
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.
Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.