The United States Preventive Services Task Force (USPSTF) recently released draft recommendations on breast cancer screening, which could be finalized within the next few months.1 The last time the Task Force (TF) weighed in on this topic was in 2009, just as the Affordable Care Act (ACA) was being debated. At that time, the TF recommendations were so controversial that Congress specified in the ACA that they should not be used to determine insurance coverage (more on this later).
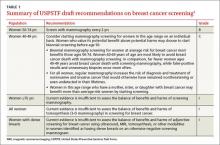
The draft recommendations (TABLE 1)1 carry a C grade for women ages 40 to 49 years (ie, offer or provide screening mammography for selected patients depending on individual circumstances) and a B grade for biennial screening of women ages 50 to 74. The proposed recommendations are basically the same as the ones made in 2009, with more detailed wording to explain the rationale for the C recommendation, and to address 2 new issues: tomosynthesis (3-D mammography) and adjunctive screening for women with dense breasts. The previous D recommendation against self breast examination was left unchanged.
Benefit of mammography screening varies by decade of life
Breast cancer is the leading cause of non-skin cancers in women and, after lung cancer, the second leading cause of cancer deaths in women. In 2014 there were 233,000 new cases diagnosed and 40,000 breast cancer deaths.1,2 While the TF found that mammography reduces deaths from breast cancer in women between the ages of 40 and 74, women ages 40 to 49 benefit the least; those ages 60 to 69 benefit the most.1,3
If 10,000 women are screened routinely for 10 years, 4 breast cancer deaths will be prevented in those ages 40 to 49, 8 in those 50 to 59, and 21 in those 60 to 69.1 And harms appear to be higher in the younger age group. TABLE 21,3 shows some of the harms resulting from one-time mammography screening of 10,000 women in each age group. Notice the benefits listed previously are from repeated screenings over a 10-year period and the harms in TABLE 21,3 are from a single mammogram.
The total benefits and harms of biennial screening in 1000 women starting at age 40 (vs age 50) include 8 cancer deaths prevented (vs 7) with a cost of 1529 false positive tests (vs 953); 204 unnecessary breast biopsies (vs 146); and 20 overdiagnoses (vs 18). However, the confidence intervals on these estimates are wide, and in each case, they overlap between the 2 groups.1
The TF recommended biennial screening for women between the ages of 50 and 74 because observational studies and modeling show no clear benefit with annual screening vs every 2 years, while annual screening results in more false positives and biopsies.
Overdiagnosis may occur in nearly 20% of cases
The potential for overdiagnosis and overtreatment is increasingly recognized as a harm of cancer screening. Overdiagnosis results from detecting a tumor during screening that would not have been detected otherwise and that would not have caused death or disease but is treated anyway. This sometimes occurs with the detection of early tumors that would not have progressed or would have progressed slowly, not causing health problems before the woman dies of other causes.
The TF is one of the only organizations that considers the potential harmful effects of this problem. While it is not possible to know for certain the rate of overdiagnosis that occurs with cancer screening, high-quality studies indicate it is close to 20% for breast cancer.3
Guidance regarding women ages 40 to 49
The new draft recommendations carefully point out that, while the overall benefit of screening women ages 40 to 49 is small, the decision to begin screening before age 50 should be an individual one, and an informed one. They state that women who value the small potential benefit over the potential for harm may choose to be screened, as might women who have a family history of breast cancer. And the recommendations do not apply to women who have a genotype that places them at increased risk for breast cancer.
Tomosynthesis: Evidence of benefit is insufficient
Tomosynthesis as a primary breast cancer screening tool was studied in a separate evidence report commissioned by the TF.4 While tomosynthesis, compared with routine mammography, appears to have increased sensitivity and specificity in detecting breast cancer, no studies looked at this technology as a primary screening tool and its effect on breast cancer mortality, overall mortality, and quality of life. Sticking to its nationally-recognized methodological rigor, the TF states that information at this time is insufficient to make a recommendation on the use of tomosynthesis.