PRACTICE RECOMMENDATIONS
› Perform laboratory testing for chronic kidney disease (CKD)-induced bone disease at CKD stage 3. B
› Avoid calcium-based phosphate binders in patients with known vascular calcifications. B
› Consider the use of phosphate binders in non-dialysis patients on a case-by-case basis, particularly in those with hyperphosphatemia not controlled by dietary measures. B
› Prescribe native vitamin D (ergocalciferol or cholecalciferol) to patients with CKD stages 3 to 4 who have secondary hyperparathyroidism and vitamin D deficiency. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 14% of the US general population has chronic kidney disease (CKD).1 Limited data exist regarding the exact prevalence of CKD-mineral and bone disorder (MBD), but abnormal mineral metabolism is believed to start in stage 3 CKD, implying that 8% of the adult US population could be at risk for, or already have established, CKD-MBD.2 Although the disorder has traditionally been managed by nephrologists, this earlier onset suggests that many patients should be screened and treated by their primary care physicians.
Because CKD-MBD can lead to significant morbidity (ie, increased fracture risk) and mortality, identification and treatment are of utmost importance.3 This review provides information from the current literature and the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, and focuses primarily on the non-dialysis CKD population.
CKD-MBD: A broad spectrum of disorders
CKD-MBD is defined as a systemic disorder of mineral and bone metabolism due to CKD. Traditionally referred to as renal osteodystrophy, the term CKD-MBD is meant to indicate and describe a broad clinical spectrum of CKD-associated bone mineral metabolism disorders that manifest from one or a combination of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.4
Renal bone disease can be divided into low bone turnover (adynamic bone disease) and high bone turnover states. Both can lead to a decrease in bone strength and an increase in pathological fractures.5
Pathophysiology: Difficult to know where the cascade begins
Understanding the pathophysiology and treatment of bone disease in patients with CKD can be challenging. Because of abnormalities of mineral metabolism and changes in hormones and cytokines, bone remodeling is severely disrupted in patients with CKD, and it remains unclear where this cascade begins.
As an adaptive response to decreased kidney function, PTH levels increase. Elevations of both fibroblast growth factor 23 (FGF23) lower blood phosphate levels by inhibiting phosphate reabsorption in the kidneys, thus increasing urinary excretion of phosphorus. Secondary hyperparathyroidism (SHPT), driven by hypocalcemia, responds to normalize serum calcium levels by increasing the number and size of osteoclasts actively breaking down bone matrix. This escalates fracture risk. In addition, the inability of damaged kidneys to convert vitamin D to an active form further deranges calcium and phosphate homeostasis.
Successful management of serum levels begins with monitoring
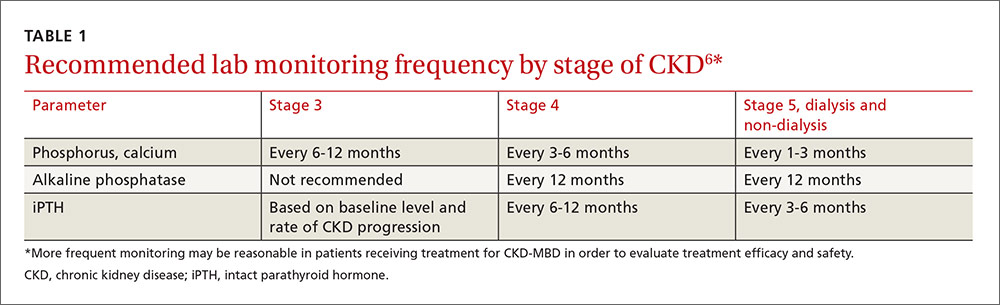
KDIGO, an independent nonprofit foundation that seeks to improve the care and outcomes of kidney disease patients worldwide, developed guidelines for the diagnosis, evaluation, prevention, and treatment of CKD-MBD in 2009.6 These guidelines recommend that treatment of CKD-MBD be aimed at managing serum phosphate, PTH, and calcium levels. The recommended frequency for laboratory monitoring of these levels varies by stage of CKD and is described in TABLE 1.6 (For more on chronic kidney disease staging, see KDIGO’s 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.)
Because of the interrelated nature of these minerals and hormones, drug therapy aimed at treating one may impact the others. This must be considered when designing treatment regimens.
Hyperphosphatemia: Manage with diet, drugs, dialysis
Observational studies have shown an association between higher serum phosphate levels and mortality.6-8 KDIGO recommends maintaining serum phosphorus levels within the normal range of the assay in patients with CKD who are not receiving dialysis.6 For dialyzed patients, the recommendation is to lower the phosphorus level toward the normal range as much as possible.6 Maintaining an appropriate phosphorus level is accomplished through dietary phosphate restriction, the use of phosphate binders, and, in dialyzed patients, dialytic removal of phosphate.6