CHICAGO – Sometimes, the skin can provide the first clues that a patient has been exposed to a drug product that has been adulterated or an over-the-counter product illegally sold in this country that contains a prescription medication, according to pediatric dermatologist Scott Norton, MD.
Speaking at the World Congress of Pediatric Dermatology, he reviewed some of the reactions associated with exposure to counterfeit drugs, contraband drugs, as well as products, misrepresented as drugs that do not include any active pharmaceutical ingredients. The worldwide market for these products is a “hugely profitable industry,” and the scope of the problem should not be underestimated, said Dr. Norton, chief of dermatology at Children’s National Health System, Washington.
Examples include completely fraudulent formulations, from fake vaccines being distributed in China and Nigeria and counterfeit antimalarials in Cambodia to “contraceptive” pills in Brazil that contain no contraceptive ingredients. In addition, many Asian so-called herbal remedies are actually adulterated with corticosteroids, antibiotics, and other prescription medications. Another issue, he noted, is that substandard medications might contain too much or too little of the active ingredient listed on the label, including adulterated vehicles or fillers. In other cases, they may be contaminated, as happened with the CNS-injectable steroids that caused serious fungal infections and deaths in the United States.It’s particularly important to have a high index of suspicion for such products given an increasingly mobile worldwide population. Today, patients and their family members who travel out of the country – and even local shopkeepers – may bring in these sorts of products from outside the United States, many of which would require a prescription in the United States.
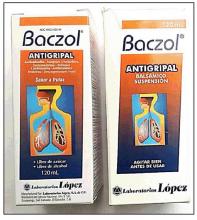
In the United States, there have been several reports of a mysterious fixed drug eruption in patients reported to have taken Baczol, a cold and flu remedy available over the counter in El Salvador for upper respiratory infections. Two of the ingredients listed on the Baczol label are sulfamethoxazole and trimethoprim, two prescription antibiotics. After determining that two Salvadoran American children with a suspected fixed drug eruption had taken a Baczol product, Dr. Norton, with the aid of medical students, was able to find Baczol containing trimethoprim-sulfamethoxazole for sale over the counter in more than one-third of the shops visited in the greater Washington area (MMWR Morb Mortal Wkly Rep. 2013 Nov 22;62[46]:914-6). Eventually, the Food and Drug Administration issued a consumer alert regarding certain Baczol products containing these ingredients, but Dr. Norton said he is still concerned about the possibility for more grave hypersensitivity reactions to these sulfa antibiotics in the Salvadoran product.
Sometimes, said Dr. Norton, the problem lies in the lack of an expected ingredient. He and his team at Children’s National Health System helped solve a medical mystery involving a skin ailment in very premature infants with cholestasis. An interdisciplinary team was convened after the neonatal intensive care unit at the hospital saw its third infant with severe blistering and erosions in an acral, perianal, and perioral pattern that did not respond to empiric treatment for herpes simplex virus and staphylococcal infection – a pattern reminiscent of zinc deficiency dermatitis. Dietitians reported that there was a nationwide shortage of sterile injectable zinc, so total parenteral nutrition was being formulated without zinc. All three of the premature infants were receiving total parenteral nutrition and were so premature that they had insufficient zinc stores. The problem was identified and corrected (MMWR 2014 Jan. 17;63[02];35-7).
A more pervasive issue, which has global significance, pertains to counterfeit vaccines prepared with absolutely no vaccine components, often made in China or Nigeria with high-quality and sophisticated packaging, said Dr. Norton.
Keeping a lid on counterfeit drugs is challenging since there are so many potential entry points into the supply chain, Dr. Norton pointed out. Weak points include mislabeled raw ingredients, packaging, storage, transportation, repackaging, and distribution. The proliferation of online pharmacies also makes regulation more difficult.
There is some international cooperation to detect and combat drug counterfeiting and adulteration: For example, Interpol, the International Coalition of Medicines Regulatory Authorities, the Pharmaceutical Security Institute, and even the United Nations are developing cooperative strategies to combat the problem.
In the meantime, he emphasized that physicians must maintain a high index of suspicion and keep in mind that the first signs of adulterated drugs or prescription drugs available OTC may appear on the skin.
Dr. Norton reported no conflicts of interest.
On Twitter @karioakes