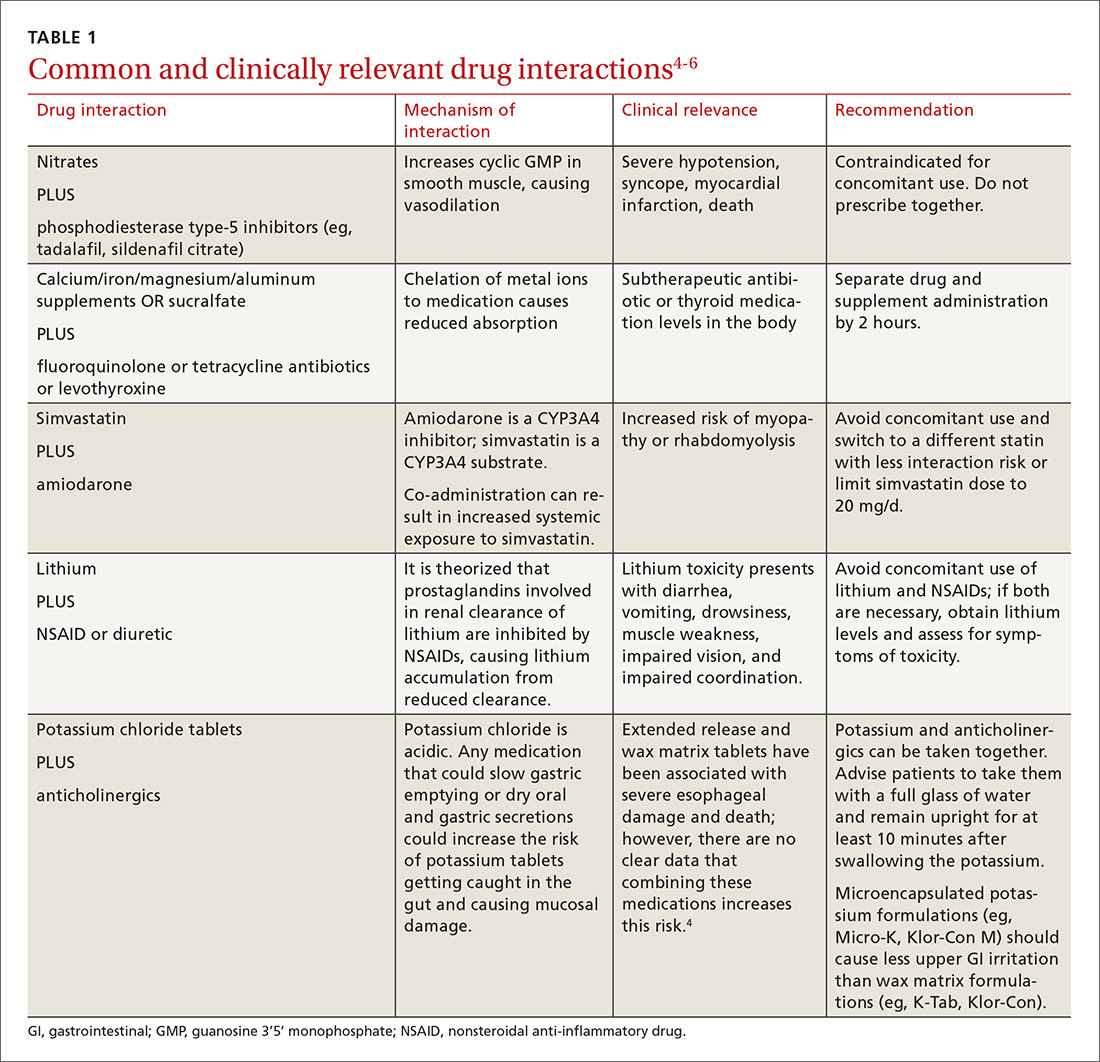
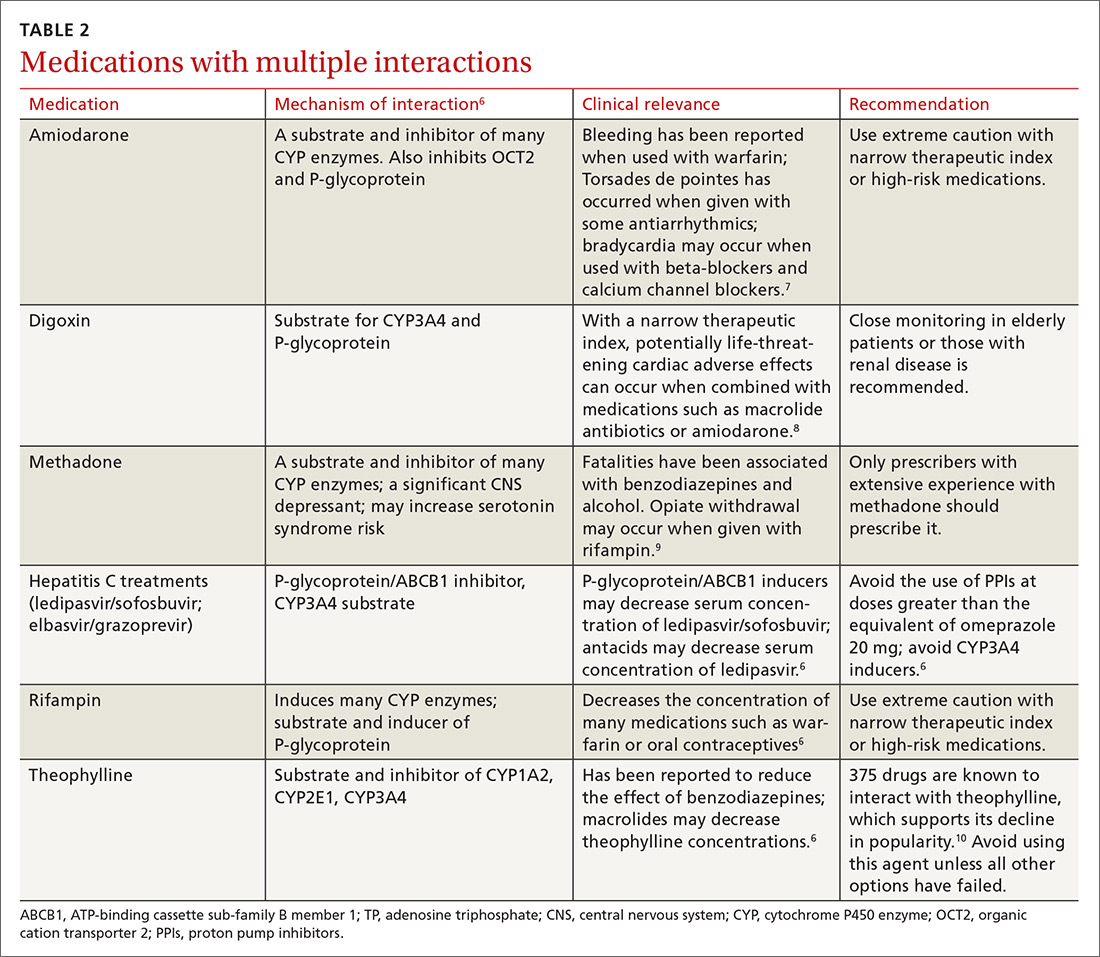
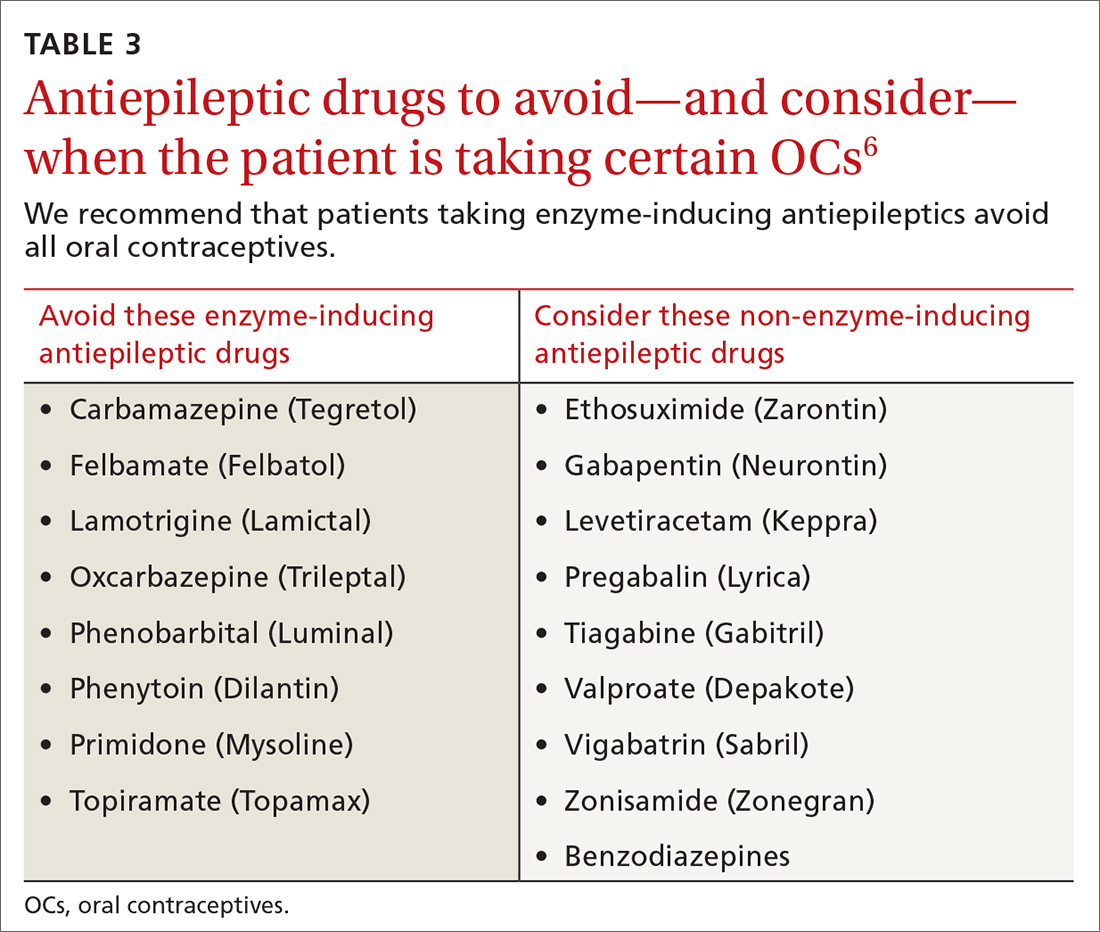
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26