Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
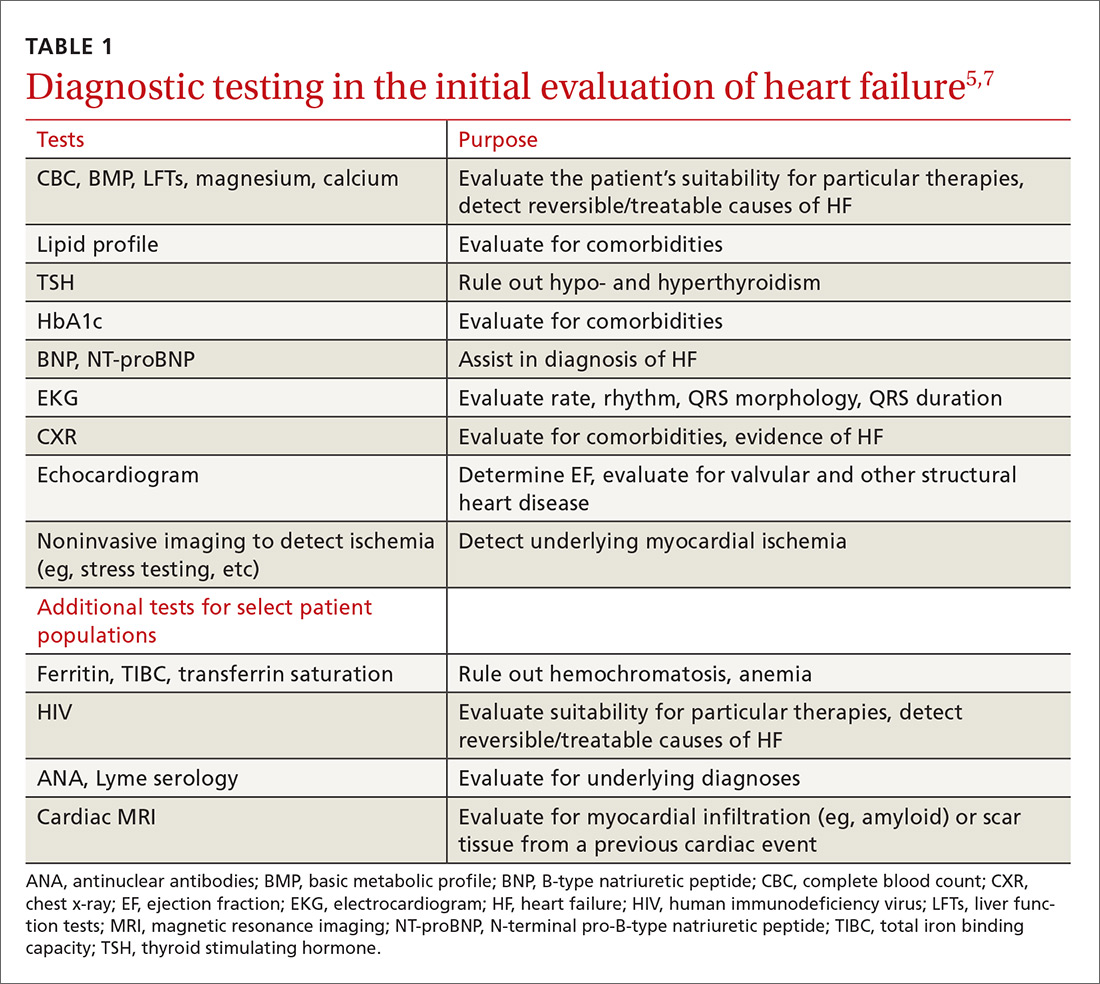
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.
Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5