Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
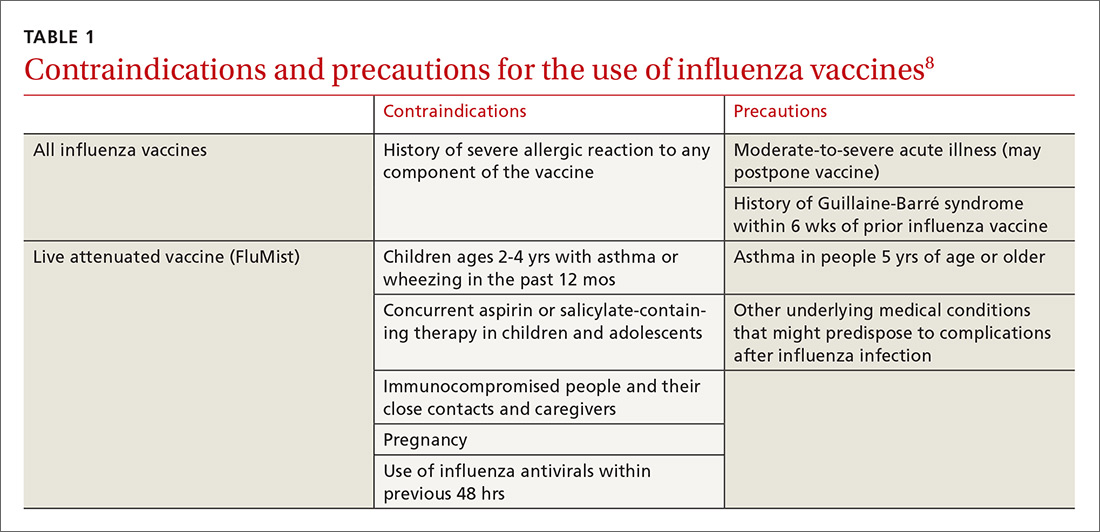
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).
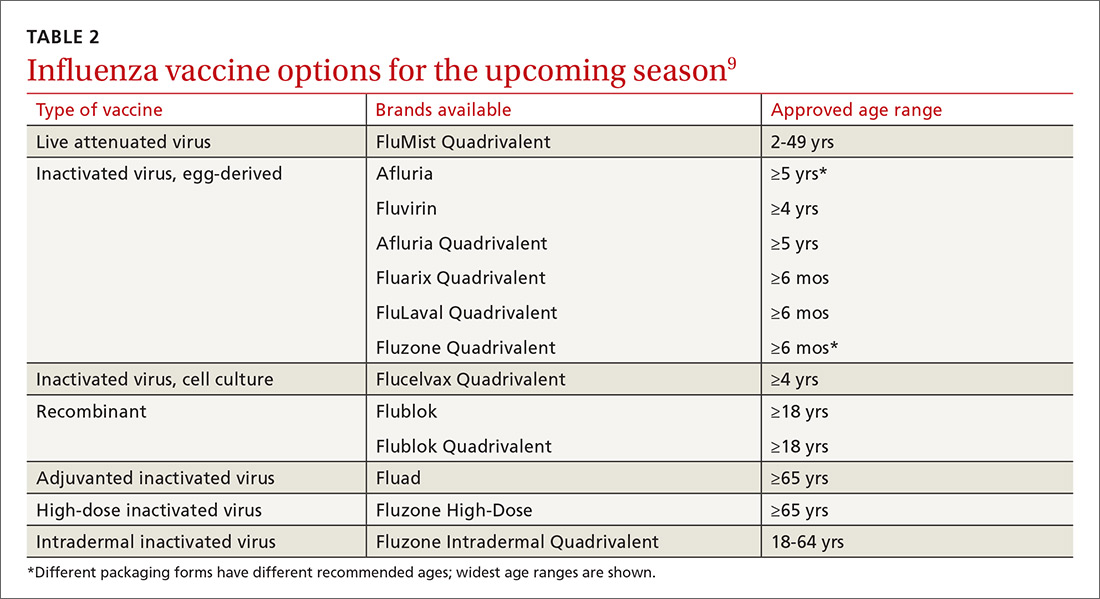
Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.
Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
Continue to: Strategy 2