Two pneumococcal vaccines are licensed for use in the United States: the 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13, Wyeth]) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23 [Pneumovax, Merck]). The recommendations for using these vaccines in adults ages ≥ 19 years are arguably among the most complicated and confusing of all vaccine recommendations made by the Advisory Committee on Immunization Practices (ACIP).
In June 2019, things got even more complicated with ACIP’s unusual decision to change the previous recommendation on the routine use of PCV13 in adults ≥ 65 years. The new recommendation states that PCV13 should be used in immunocompetent older adults only after individual clinical decision making. The recommendation for routine use of PPSV23 remains unchanged. This Practice Alert explains the reasoning behind this change and its practical implications.
How we got to where we are now
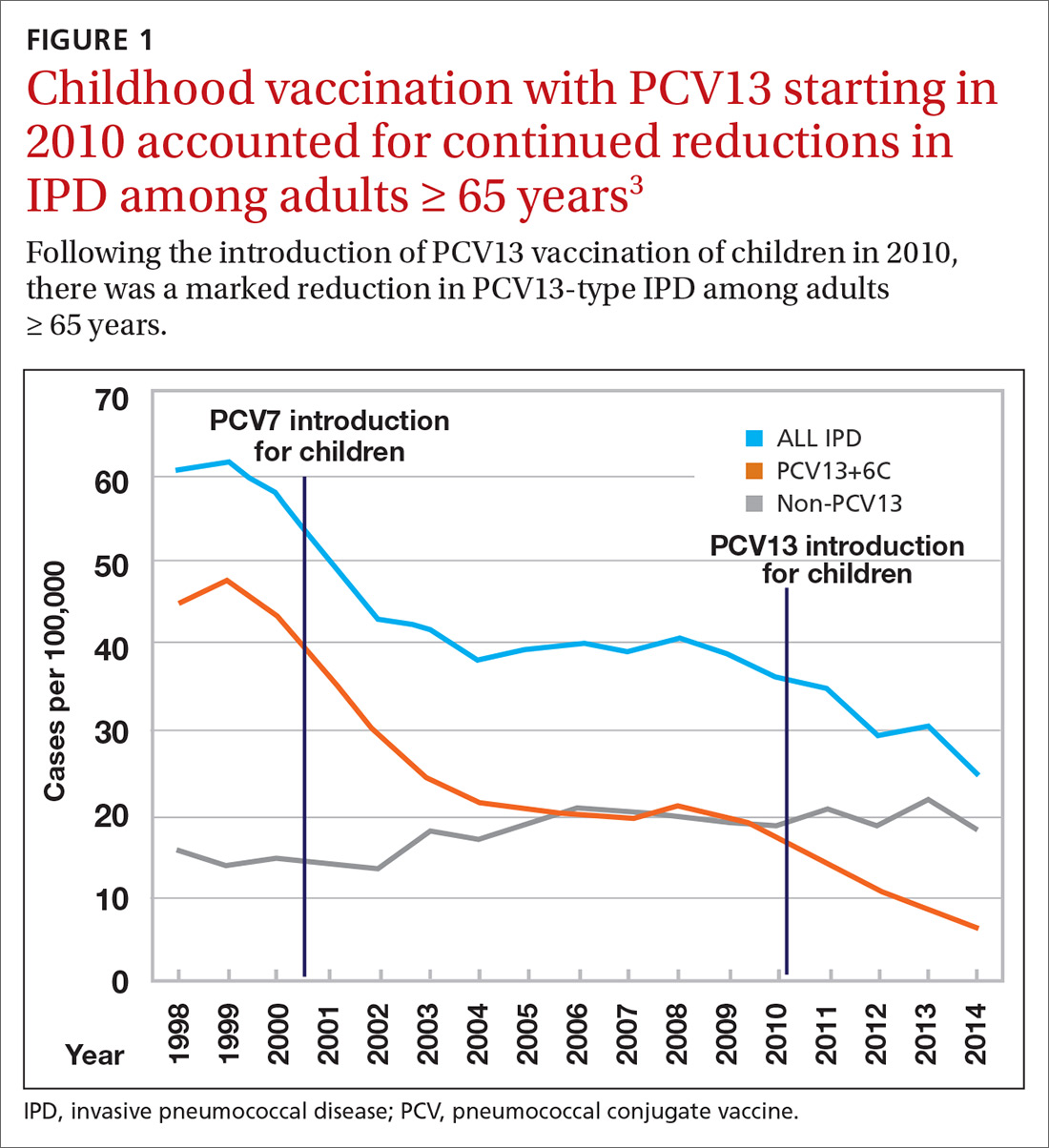
Nearly 20 years ago, PCV was introduced into the child immunization schedule in the United States as a 7-valent vaccine (PCV7). In 2010, it was modified to include 13 antigens. And in 2012, the use of PCV13 was expanded to include adults with immunocompromising conditions.1 In 2014, PCV13 was recommended as an addition to PPSV23 for adults ≥ 65 years.2 However, with this recommendation, ACIP noted that the incidence of invasive pneumococcal disease in the elderly had been declining since the introduction of PCV7 use in children in the year 2000 (FIGURE 13), presumably due to the decreased transmission of pneumococcal infections from children to older adults.
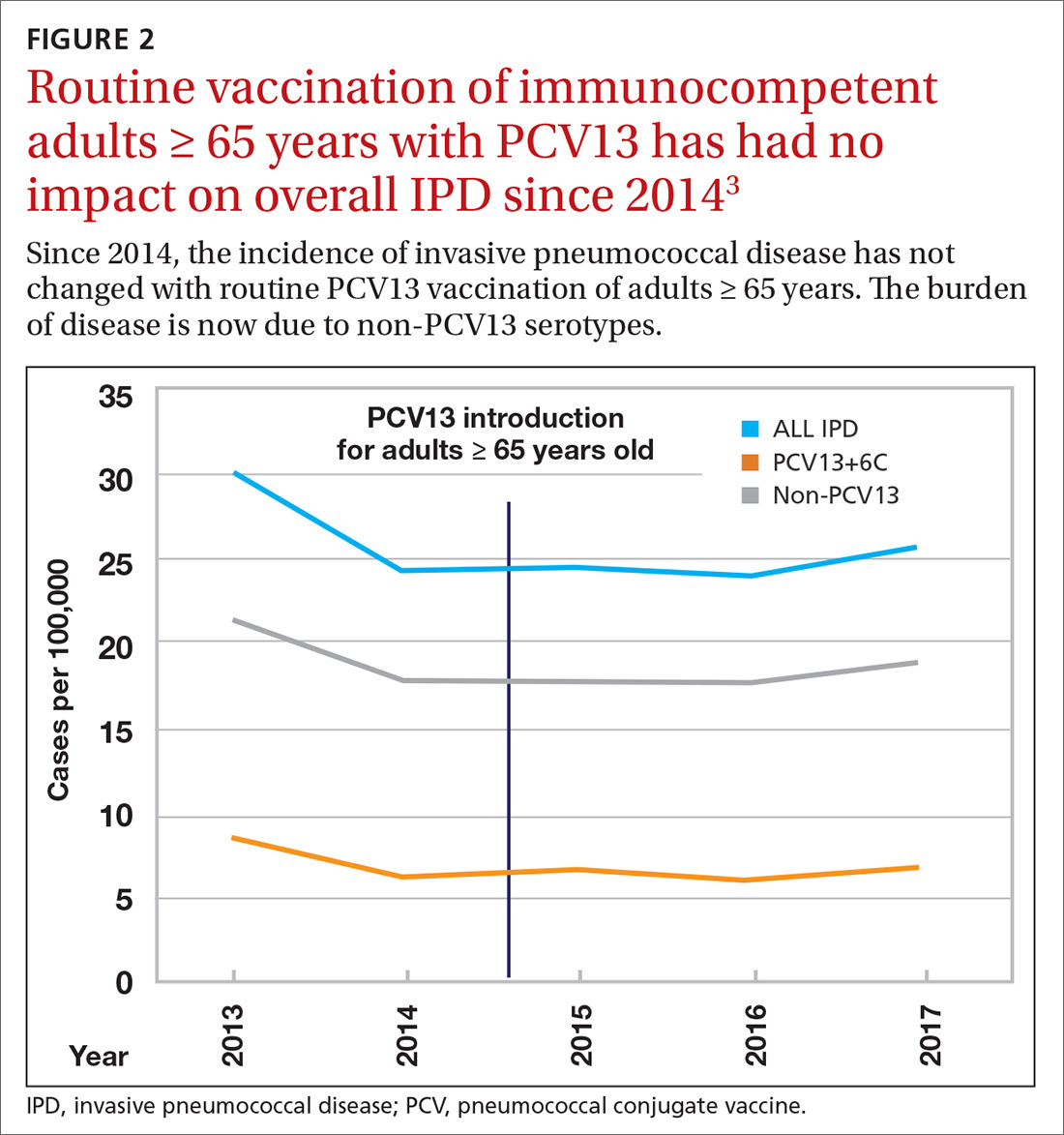
Because it was unclear in 2014 how much added benefit PCV13 would offer older adults, ACIP voted to restudy the issue after 4 years. At the June 2019 ACIP meeting, the results of an interim analysis were presented. ACIP concluded that routine use of PCV13 in immunocompetent adults ≥ 65 years adds little population-wide public health benefit given the vaccine’s routine use among children and immunocompromised adults (FIGURE 23).
ACIP had 3 options in formulating its recommendations.
- Recommend the vaccine for routine use universally or among designated high-risk groups.
- Do not recommend the vaccine.
- Recommend the vaccine only for specific patients after individualized clinical decision making.
The last option—the one ACIP decided on—applies when a safe and immunogenic vaccine has been approved by the Food and Drug Administration and may be beneficial for (or desired by) individuals even though it does not meet criteria for routine universal or targeted use.
Practical issues
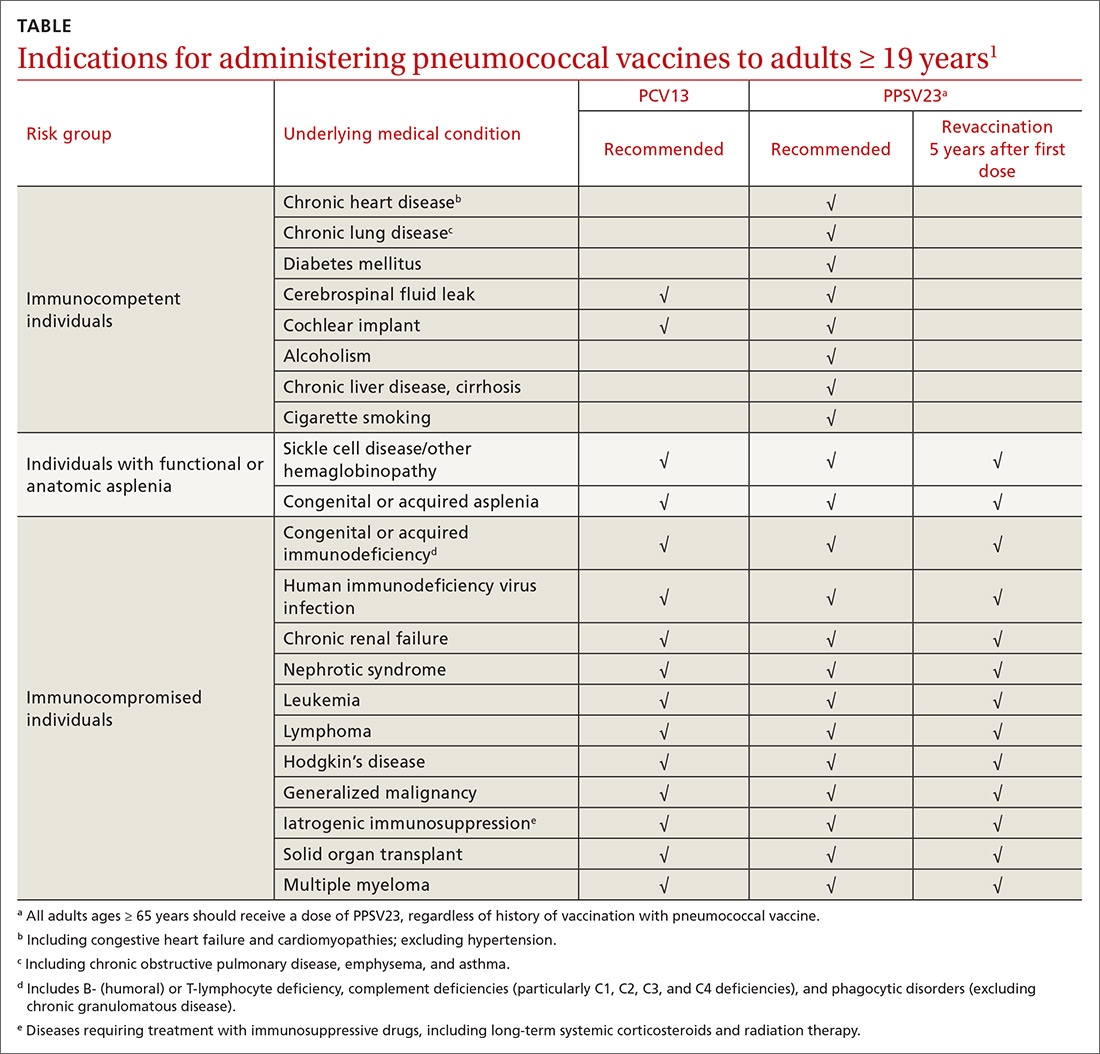
ACIP recommendations for the use of PCV13 and PPSV23 in adults vary according to 3 categories of health status: immunocompetent patients with underlying medical conditions; those with functional or anatomic asplenia; and immunocompromised individuals (TABLE1). Those in the latter 2 categories should receive both PCV13 and PPSV23 and be revaccinated once with PPSV23 before the age of 65 (given 5 years after the first dose). For immunocompetent individuals with underlying medical conditions, only those with cerebral spinal fluid leaks or cochlear implants should receive both PCV13 and PPSV23, although revaccination with PPSV23 before the age of 65 is not recommended.
Continue to: Prior to the recent change...