Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
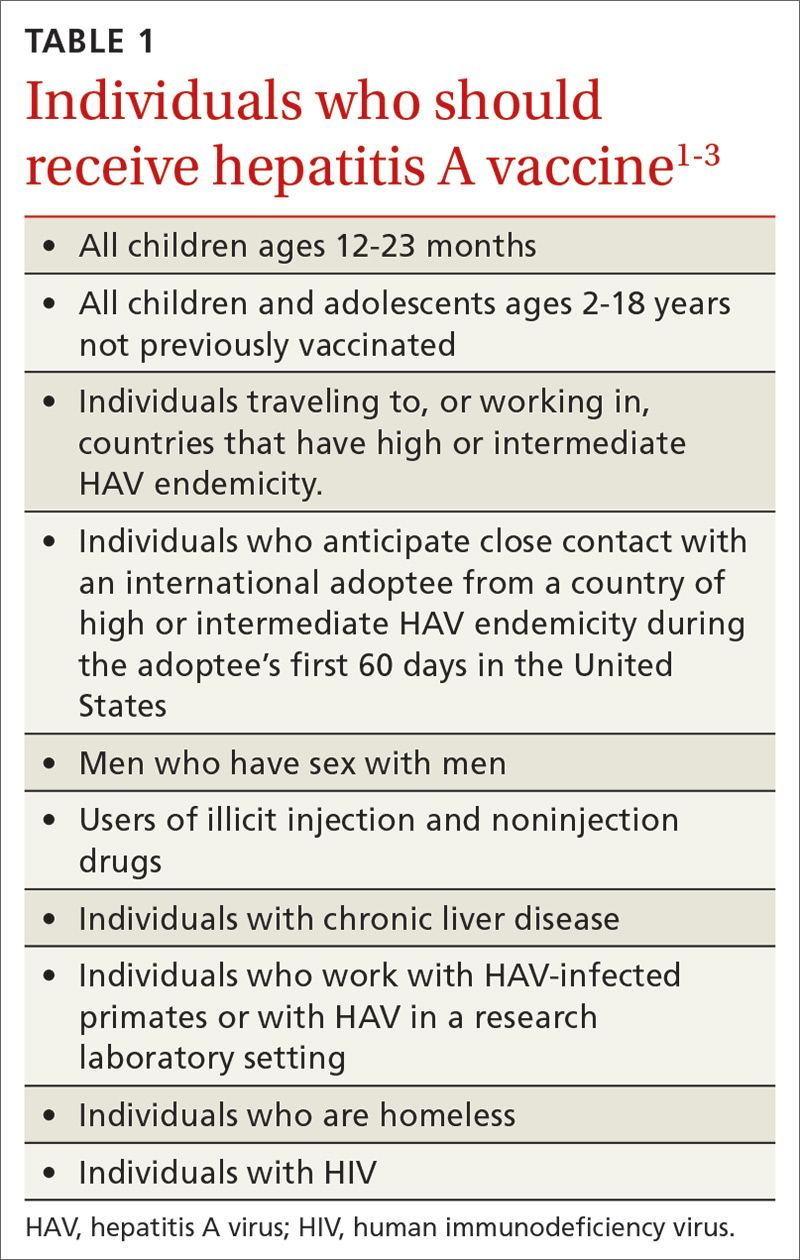
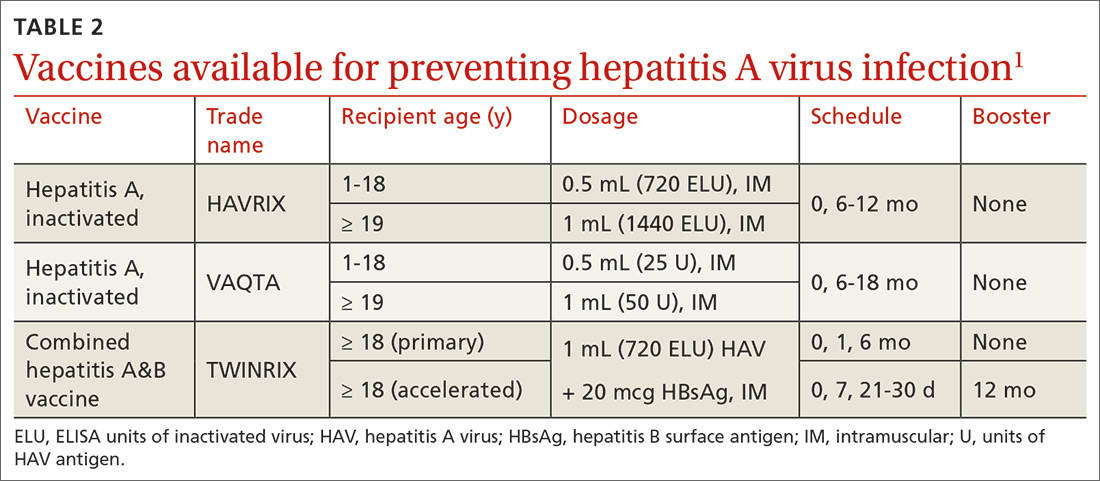
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1
ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2
At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...