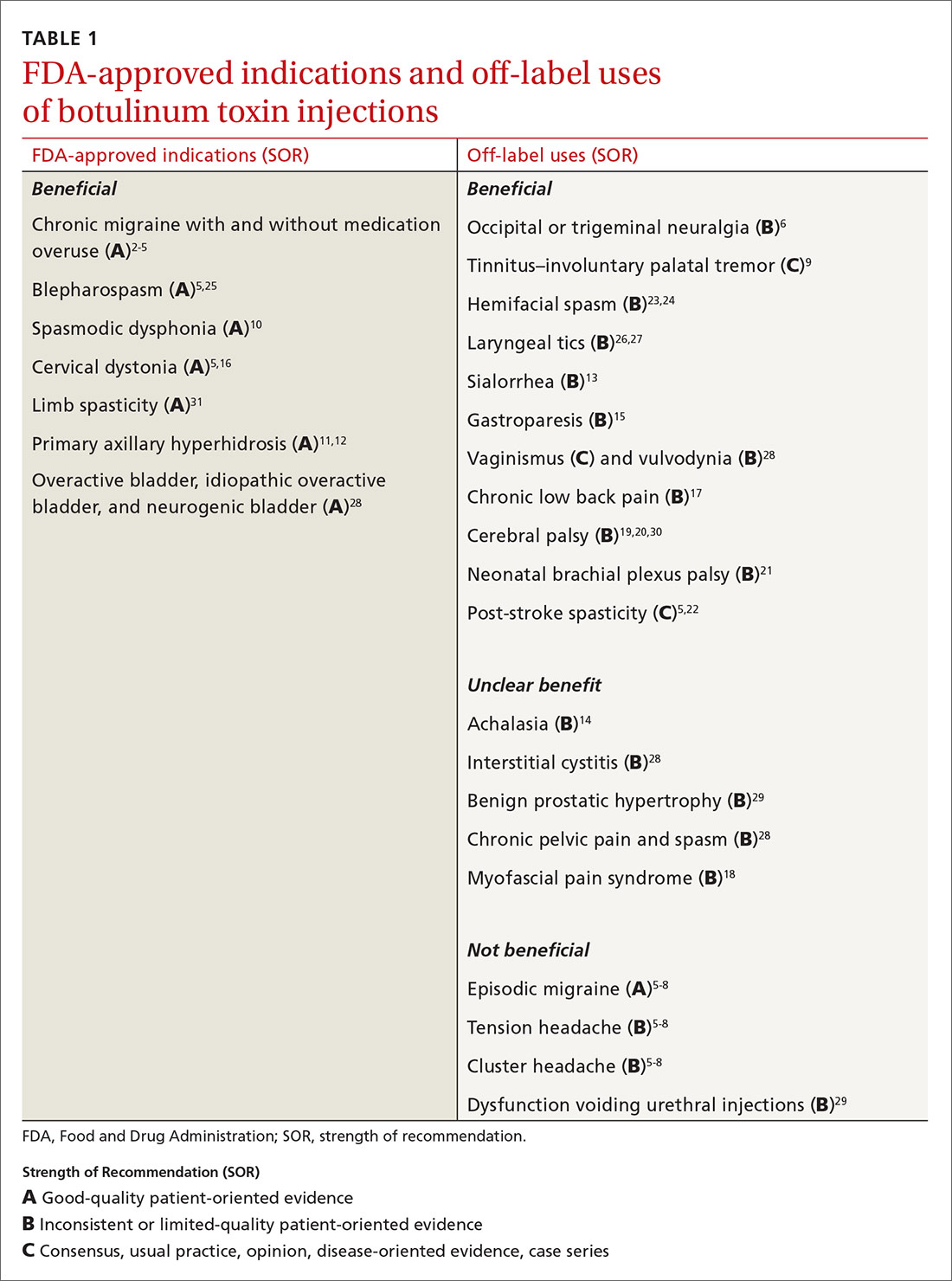
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.
But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).
Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches