Dupilumab (Dupixent, Sanofi and Regeneron) significantly reduced exacerbations compared with placebo in children ages 6-11 years who had moderate-to-severe asthma in a phase 3 trial.
A fully human monoclonal antibody, dupilumab also improved lung function versus placebo by week 12, an improvement that lasted the length of the 52-week trial.
Dupilumab previously had been shown to be safe and effective in adolescents and adults with moderate-to-severe asthma, patients 6 years and older with moderate-to-severe atopic dermatitis, and adults with chronic rhinosinusitis with nasal polyposis, but its safety and effectiveness for moderate-to-severe asthma in the 6-11 years age group was not known.
Results from the randomized, double-blind VOYAGE study conducted across several countries were presented Saturday, July 10, at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021.
Leonard B. Bacharier, MD, professor of pediatrics, allergy/immunology/pulmonary medicine at Vanderbilt University Medical Center in Nashville, Tennessee, presented the results from the trial, which was funded by Sanofi/Regeneron.
Researchers enrolled 408 children ages 6-11 years with uncontrolled moderate-to-severe asthma. Children on high-dose inhaled corticosteroids (ICS) alone or medium-to-high–dose ICS with a second controller were randomly assigned either to add-on subcutaneous dupilumab 100 mg or 200 mg, based on body weight at study start, or to placebo every 2 weeks for 52 weeks.
Analyses were done in two populations: 350 patients with markers of type 2 inflammation (baseline blood eosinophils ≥150 cells/μl or fractional exhaled nitric oxide [FeNO] ≥20 ppb) and 259 patients with baseline blood eosinophils ≥300 cells/µl.
“The primary endpoint was the annualized rate of severe asthma exacerbations,” Dr. Bacharier said. “The key secondary endpoint was change in percent predicted prebronchodilator FEV1 [forced expiratory volume at 1 second] from baseline to week 12.”
At week 12, the annualized severe asthma exacerbation rate was reduced by 59% (P < .0001) in children with blood eosinophils ≥300 cells/µL and results were similar in those with the type 2 inflammatory phenotype compared with placebo.
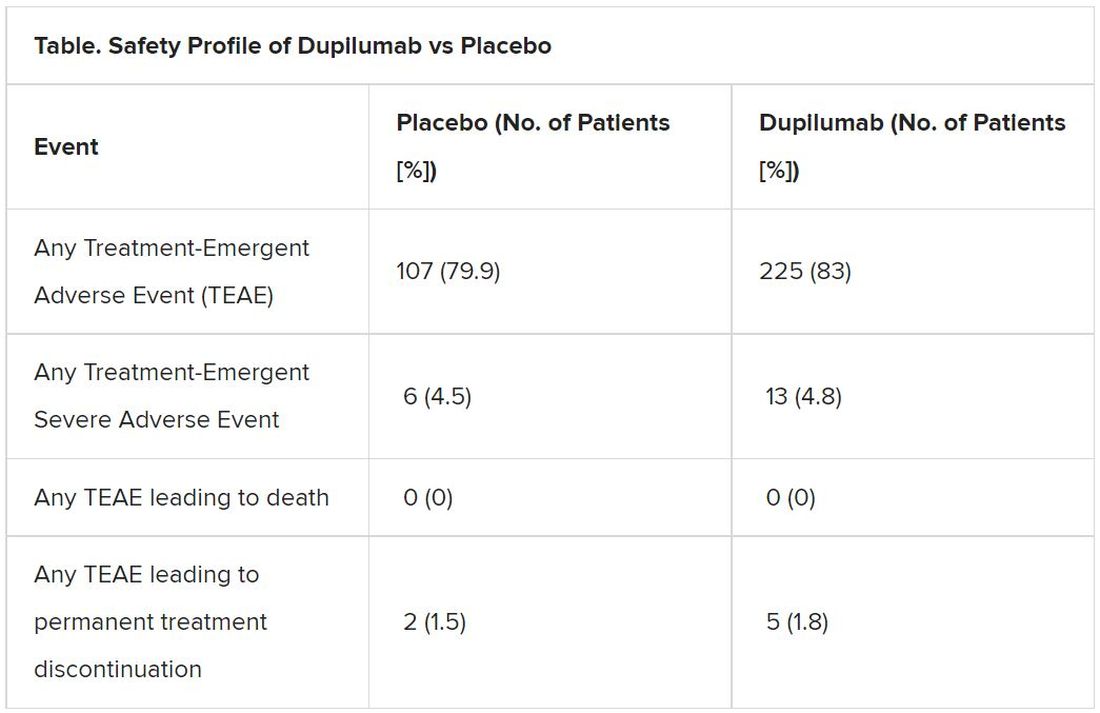
Results also indicate a favorable safety profile for dupilumab.
James M. Tracy, DO, an expert with the American College of Allergy, Asthma, and Immunology, told this news organization that adding the dupilumab option for children in the 6-11 age group is “huge.”
Dr. Tracy, who was not involved with the study, said although omalizumab (Xolair, Genentech) is also available for these children, dupilumab stands out because of the range of comorbidities it can treat.
“[Children] don’t have the same rhinosinusitis and polyposis that adults would have, but a lot of them have eczema, and this drug with multiple prongs is incredibly useful and addresses a broad array of allergic conditions,” Dr. Tracy said.
More than 90% of children in the study had at least one concurrent type 2 inflammatory condition, including atopic dermatitis and eosinophilic esophagitis. Dupilumab blocks the shared receptor for interleukin (IL)-4/IL-13, which are key drivers of type 2 inflammation in multiple diseases.
Dr. Tracy said that while dupilumab is not the only drug available to treat children 6-11 years with moderate-to-severe asthma, it is “a significant and unique addition to the armamentarium of the individual practitioner taking care of these very severe asthmatics in the 6-11 age group.”
Dupilumab also led to rapid and sustained improvement in lung function. At 12 weeks, children assigned dupilumab improved their lung function as measured by FEV1 by 5.21% (P = .0009), and that continued through the 52-week study period.
“What we know is the [improved lung function] effect is sustained. What we don’t know is how long you have to keep on the drug for a more permanent effect, which is an issue for all these biologics,” Tracy said.
Dr. Bacharier reported speaker fees and research support from Sanofi/Regeneron. Dr. Tracy has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.