The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
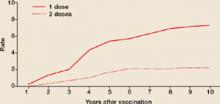
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.