Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
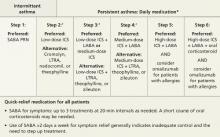
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.