The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
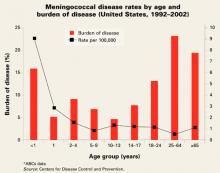
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.