› Evaluate patients for movement disorders before initiating or adjusting antipsychotic therapy, then weekly until the dose is stabilized. A
› Use nonpharmacologic interventions—eg, positive reinforcement, music, light exercise—as first-line therapy for neuropsychiatric symptoms of dementia; consider antipsychotic therapy only if they fail. A
› Obtain a fasting glucose level before initiating or adjusting antipsychotic therapy, then at 12 weeks, and annually if the patient is taking a second-generation agent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve B is a 43-year-old patient with bipolar disorder and a history of hypertension and high cholesterol. His body mass index (BMI) is 29. During a checkup, he tells you his psychiatrist recently started him on olanzapine. He reports that the medication is working, but he’s concerned about adverse effects, and asks whether he should be monitored for signs of diabetes.
CASE 2 › Mary F, an 83-year-old with Alzheimer’s disease and a history of stable coronary artery disease, is a resident in a long-term care facility, where staff members report that she is increasingly combative. The floor nurse says Ms. F has been striking out at the nurses’ aides who attempt to dress her and asks that you prescribe an antipsychotic to “calm her down.”
If Mr. B and Ms. F were your patients, what would you do?
In 1951, the chance discovery of an anesthetic’s calming properties was the first step in the development of the medications that came to be known as antipsychotics.1 In recent years, we have seen an expansion in both the number of antipsychotic agents on the market and the scope of their use, for conditions as varied as chronic pain, dementia, nausea and vomiting, and Tourette syndrome.
While antipsychotics often are prescribed by psychiatrists or other specialists, primary care physicians are increasingly likely to be involved in the management of patients who take them—and, at times, to prescribe antipsychotic agents themselves. We developed this guide to increase awareness of safe prescribing practices and principles guiding the initiation and management of antipsychotic agents. We start with a review of the mechanism of action of first- and second-generation antipsychotics (SGAs).
In the last decade, research has called into question whether second-generation antipsychotics really are more effective than first-generation agents
First- and second-generation agents: How they work and differ
Antipsychotics act at the level of the dopaminergic pathways in the central nervous system by blocking the D2 receptors. Action on the mesolimbic pathway is thought to be responsible for their effects on schizophrenia symptoms,2 while action at receptor sites in other dopaminergic pathways leads to common adverse effects, primarily the extrapyramidal symptoms (EPS) associated with first-generation antipsychotics (FGAs).
The distinction between first- and second-generation agents relates to SGAs’ blockage of serotonin receptors (thought to better relieve schizophrenia symptoms) and increased specificity for the mesolimbic pathway (which reduces the action on other dopamine pathways and is less likely to produce EPS).3 These differences largely accounted for the belief that SGAs were more effective and provided the rationale for their designation as atypical antipsychotics.
Are SGAs really better?
In the last decade, research has called such claims into question. Trials such as the Clinical Antipsychotic Trials of Intervention Effectiveness4 and Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia study,5 as well as a meta-analysis,6 found that SGAs as a class are no more effective than FGAs. That said, 2 SGAs—clozapine and olanzapine—were found to be superior to FGAs for the treatment of schizophrenia. The studies also raised doubts about SGAs’ advantages regarding tolerability, as the time to discontinuation due to intolerable adverse effects was similar for first- and second-generation drugs.4-6
Approved and off-label indications: A look at the evidence
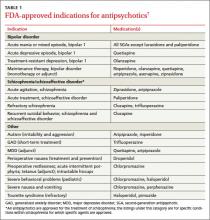
In addition to schizophrenia, many antipsychotics have US Food and Drug Administration (FDA) approval to treat various psychiatric and nonpsychiatric conditions (TABLE 1).7 Several are approved for use in bipolar disorder, 2 are approved as adjunctive treatment of major depressive disorder (MDD), and one is approved for the short-term treatment of generalized anxiety disorder (GAD). Porphyria, tetanus, and intractable hiccups are among the nonpsychiatric conditions for which some antipsychotics are approved.7
Evidence ranging from anecdotal to randomized controlled trials (RCTs) is steadily emerging about off-label uses of antipsychotics, with risperidone, quetiapine, and olanzapine foremost among them.8,9 Use of antipsychotics in the treatment of neuropsychiatric symptoms (NPS) of dementia has become particularly widespread, with off-label use of antipsychotics more prevalent in long-term care facilities than in outpatient settings.9