The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) released several new immunization recommendations in 2013, and they pertain to special populations: infants at high risk for meningococcal disease who could benefit from a new vaccine; children ages 2 to 16 years who may now receive the Japanese encephalitis (JE) vaccine if traveling to Asia; and children and adolescents at high risk for Streptococcus pneumoniae infections who should receive the 13-valent pneumococcal conjugate vaccine (PCV13).
New meningococcal vaccine for high-risk infants
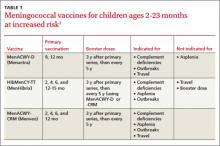
A third vaccine against meningococcal disease, Menveo (Novartis), has been approved for use in high-risk infants ages 2 to 23 months—those with complement deficiencies or functional or anatomic asplenia, those involved in an institutional or community outbreak of meningococcal disease, and those traveling to a meningococcal-endemic area (eg, the meningitis belt of Africa). The 3 vaccines approved for use in infants (TABLE 1) vary in indications and use for travel.1 Keep in mind that vaccination against meningococcal disease is not recommended for routine use in infants because of the low prevalence of disease and a predominance of meningococcal type B, which is not prevented by any of the current vaccines.2
Japanese encephalitis vaccine approved for children, adolescents
Since 2009, a vaccine of inactivated JE virus derived from Vero cell culture (Ixiaro, manufactured by Intercell Biomedical and distributed by Norvartis) has been available in the United States for adults ≥17 years who travel during JE virus transmission season to parts of Asia where the virus is endemic. In May 2013, the US Food and Drug Administration licensed the JE vaccine for use in children ages 2 to 16 years, and in June 2013, ACIP recommended it for those in this age group for the same indications as adults.3
Individuals at high risk are those likely to visit rural areas where the virus is prevalent. The vaccine is not recommended for short-term travelers who will confine their visit to urban areas or who will refrain from traveling during the JE transmission season. The CDC travel Web site describes the geographic areas where the JE virus can be found and the timing of the JE season (http://wwwnc.cdc.gov/travel/diseases/japanese-encephalitis; see “Who is at risk?”). Use of the vaccine will be uncommon, and most of those who will receive it will need to visit a travel clinic or local health department. A primary series is 2 doses, given 28 days apart.
Pneumococcal vaccine for high-risk children up to age 18
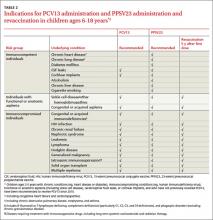
ACIP now recommends the use of both PCV13 and 23-valent pneumococcal polysaccharide vaccine (PPSV23) in children ages 6 to 18 years who have not been vaccinated with these products and who have certain immunocompromising conditions, functional or anatomic asplenia (including those with sickle cell disease), cerebrospinal fluid leaks, or cochlear implants (TABLE 2).4 PPSV23 alone is recommended for those in this age group with chronic heart, lung, or liver disease or diabetes, and for those who smoke. The recommendation is complicated because those with asplenia or immunocompromising conditions should receive 2 doses of PPSV23 at least 5 years apart (TABLE 2). PCV13 is indicated even if the patient was previously fully immunized with 7-valent pneumococcal conjugate vaccine.
Ideally, all children for whom PCV13 is indicated would receive a full vaccine series as infants, and at 2, 4, 6, and 12 to 15 months of age. Those with chronic medical conditions and immunocompromising conditions should additionally receive PPSV23 at age 2 years, and again 5 years later if they have asplenia or an immunocompromising condition.5 However, some of these children will remain unimmunized; others can become underimmunized if they acquire a condition that heightens their risk for pneumococcal disease. Both situations make necessary the new recommendation for those ages 6 to 18 years. For those who have received neither vaccine, the recommended sequence is to receive PCV13 first, followed by PPSV23 at least 8 weeks later.
Pneumococcal vaccine recommendations are now uniform for those ages 6 to 64 years with chronic medical conditions and immunocompromising conditions.2,6 All those ≥65 should receive PPSV23, even if they have received 2 previous doses. At least 5 years should have passed from a previous PPSV23 dose. This is the only scenario in which a third dose of PPSV23 is recommended.
Universal use of pneumococcal vaccines has markedly reduced invasive pneumococcal disease nationally in vaccinated and unvaccinated individuals.5 While invasive pneumococcal disease rates have fallen overall, patients with high-risk conditions still have a high relative risk. The incidence rates (cases per 100,000) for children with hematologic malignancies were estimated at 1282 (rate ratio [RR], compared with children without this condition, of 822); 197 for those with HIV infection (RR=122), and 56 for those with sickle cell disease (RR=27).4