Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The pathophysiology of type 2 diabetes mellitus (T2DM) is multifactorial and includes mechanisms beyond insulin resistance and pancreatic β-cell dysfunction
- The differential actions of glucagon-like peptide (GLP)-1 agonists and dipeptidyl peptidase (DPP)-4 inhibitors include:
- The additional actions of GLP-1 agonists on pathophysiologic mechanisms include:
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The pathophysiology of T2DM and the incretin system have been described in considerable detail in previous Journal of Family Practice supplements.1,2 In this article, we will briefly review the multiple pathophysiologic mechanisms known to be involved in T2DM, with a focus on the incretin system, to gain a better understanding of the disease progression affecting our 3 patients presented in the supplement introduction.
The central roles of insulin resistance and pancreatic β-cell dysfunction (insulin deficiency) in the pathogenesis of T2DM have been recognized for decades. But other causes of T2DM have been identified, including:
- Altered glucose release and disposal
- Altered glucagon secretion
- Impaired incretin response
- Rapid gastric emptying
- Impaired satiety
While there is a link between insulin resistance and pancreatic β-cell failure,3 the extent to which other causes interact is only beginning to be recognized. With a better understanding of the multifactorial pathogenesis of T2DM, however, has come additional treatment options and the opportunity for a more individualized and complementary approach to treatment.
Insulin resistance and pancreatic β-cell dysfunction
T2DM is a progressive disease characterized by worsening hyperglycemia,4 caused in part by insulin resistance and deterioration in pancreatic β-cell function. Insulin resistance appears to result from a complex interaction between visceral fat and the immune system that results in a state of chronic inflammation. Proinflammatory proteins secreted primarily from visceral fat block the action of insulin in adipocytes.5 Elevated levels of free fatty acids, which are common in obesity, further promote insulin resistance.6
Pancreatic β-cell dysfunction occurs progressively over a decade or more until β-cells are no longer capable of secreting sufficient insulin. Data from several studies suggest that, on average, 50% to 80% of β-cell function has been lost at the time of diagnosis of T2DM.7-9 The extent of pancreatic β-cell dysfunction is important for 2 reasons. First, medications that act by stimulating the β-cell to secrete insulin lose their effectiveness over time, as shown in the UK Prospective Diabetes Study (UKPDS)7 and, more recently, in A Diabetes Outcome Progression Trial (ADOPT).10 Second, since only 20% to 50% of pancreatic β-cell function remains at the time of diagnosis, preserving β-cell function would likely be beneficial for long-term glycemic control.
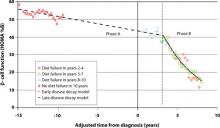
But there is no time to waste following diagnosis of T2DM. The Belfast Diet Study,8 a 10-year prospective clinical trial of 432 newly diagnosed persons with T2DM treated with intensive lifestyle management, suggested that β-cell decline occurs in 2 phases. Beginning well before diagnosis, the annual rate of decline in β-cell function during the first phase, ≥15 years prediagnosis, is about 2%, while during the second phase, beginning about 3 years postdiagnosis, the annual rate of decline in β-cell function accelerates to 18% (FIGURE 1). This second phase appears to result from a combination of steadily increasing β-cell death rate and decreasing replication rate.8
Case 2: A 47-year-old man diagnosed with T2DM several years ago who has experienced edema and weight gain on pioglitazone
Case 3: A 68-year-old woman with longstanding T2DM who has failed dual oral therapy and has microvascular complications
FIGURE 1 Phases of decline in pancreatic β-cell function8
HOMA %B, homeostasis model of assessment–percent β-cell function.
Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96(4):281-288, by permission of Oxford University Press.