Since the 1950s, researchers and clinicians have sought a simple chemical biomarker that would aid in the diagnosis and treatment of cardiovascular disease (CVD). An initial discovery was that proteins normally found within cardiac myocytes are released into the circulation when the myocardial cell membrane loses its integrity as a result of hypoxic injury.1 Over the past 60 years, major advances have been made to distinguish which proteins are highly cardiac specific, and which are suggestive of other pathologic cardiovascular processes.
In the past 15 to 20 years, significant data have emerged that support the role of cardiac-specific troponins in the cardiac disease process, making their measurement an important consideration in diagnosis and clinical decision-making for patients with various forms of CVD.2-7 With the exception of 1918, CVD has accounted for more deaths in the United States than any other illness in every year since 1900.8 It has been suggested that nearly 2,300 Americans die of CVD each day—an average of one death every 38 seconds. The direct and indirect costs of CVD for 2010 were projected at $503.2 billion—including $155 billion for total hospital costs alone.9 By comparison, a 2010 projection from the National Cancer Institute was $124.57 billion for the direct costs of cancer care.10
It is imperative for practitioners in all areas of medicine to have a working knowledge of the role of cardiac biomarkers and their potential impact on patients’ overall health. Primary care clinicians in particular should be familiar with the release kinetics of certain cardiac biomarkers to aid in the diagnosis and treatment of the cardiac patient. This article is intended as an overview of the cardiac biomarkers that are being used in practice today, and to review the novel biomarkers that may have an impact on the way clinicians practice in the near future.
BIOMARKERS CURRENTLY IN USE
Cardiac Troponins
The cardiac-specific troponins (cTn) are an integral component of the myosin-actin binding complex found in striated muscle tissue. The troponin complex includes three regulatory proteins: troponin C, troponin I, and troponin T. The genes that code for troponin C are identical in skeletal and cardiac tissue; for this reason, troponin C becomes much less cardiospecific.
However, cardiac troponin T (cTnT) and cardiac troponin I (cTnI) have differing amino acid sequences, making it possible to develop highly sensitive immunoassays to detect circulating antibody-troponin complexes after myocardial cell injury, as seen in acute coronary syndrome (ACS).12-14 No cross-reactivity occurs between skeletal and cardiac sources, indicating the unique cardiospecificity of the cTn proteins.11
Of note, there are numerous patient populations in which elevated cTn may be found; the associated conditions are outlined in Table 1.11,15,16 In particular, elevations in cTn (more specifically cTnT) are commonly observed in patients with end-stage renal disease (ESRD).11,17 The pathogenesis of elevated cTnT is not completely understood but has been proposed as a promising prognostic tool for use in patients with ESRD. Elevated levels of cTnT identify patients whose chance of survival is poor, with an increased risk for cardiac death.7 It should be noted that a significant proportion of patients with chronic renal failure succumb to cardiovascular death.17
Recently, deFilippi and colleagues18 reported a correlation between trace amounts of circulating cTnT in older patients not known to have CVD and an increased risk for future heart failure or even cardiovascular-related death. This novel finding may add valuable information to the screening and risk stratification of relatively healthy but sedentary individuals older than 65.
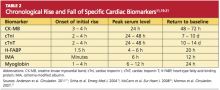
Elevations in cTn can be seen as early as two to four hours after myocardial injury.11 A small portion of cTnT and cTnI is found within the myocardial cells’ cytosol and is not bound to the troponin complex, which begins to degrade after cell injury.1,14 This cytosolic pool allows for earlier recognition of cardiac injury. As increasingly sensitive assays are developed, rises in cTn levels can be detected earlier after symptom onset, thus making the use of less specific early biomarkers, such as myoglobin and creatine kinase–MB, obsolete.
Because of the continuous release of structural proteins from the degrading myocardial tissue, elevations of cTnI may persist for seven to 10 days, while cTnT elevations can continue for 10 to 14 days postinfarction.11 This prolonged period allows for detection of even very slight cardiac damage and provides an advantage for identification and treatment of high-risk patients. When measured 72 hours after infarction, cTnI can also provide prognostic information, such as the potential size of the affected myocardial area.5,6 This, too, facilitates risk stratification. Timing of the release of key biomarkers can be seen in Table 2.11,19-21